JEE Advanced (True/False): The p-Block Elements | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Red ph osphorus is less volatile than white phosphor us because the former has a tetrahedral structure. (1982 - 1 Mark)
Ans. F
Solution. False : Red phosphorus is polymeric substance. It exist as chains of P4 tetrahedra linked together. Therefore, it is less volatile than white phosphorus.
Q.2. When PbO2 reacts with a dilute acid, it gives hydrogen peroxide. (1982 - 1 Mark)
Ans. F
Solution. False : PbO2 is a dioxide and it does not give hydrogen peroxide when it reacts with a dilute acid.
PbO2 + 4HCl → PbCl2 + Cl2 + 2H2O
Q.3. Carbon tetrachloride burns in air when lighted to give phosgene. (1983 - 1 Mark)
Ans. F
Solution. False : CCl4 gives phosgene with superheated steam
CCl4 + H2O → COCl2 + 2HCl
Q.4. Dil. HCl oxidizes metallic Fe to Fe2+. (1983 - 1 Mark)
Ans. T
Solution. True : Fe + 2HCl → FeCl2 + H2 [In FeCl2, Fe is in +2 state.]
Q.5. In aqueous solution chlorine is a stronger oxidizing agent than fluorine. (1984 - 1 Mark)
Ans. F
Solution. False : Since halogens have high electron affinities, they easily pick up electrons from other substances. Hence halogens are oxidising agents. The oxidising power decreases from fluorine to iodine. Since fluorine is the strongest oxidising agent it will oxidise any of the other halide ions in solution or when dry. Similarly, Cl2 will displace Br– and I– ions from their solutions and Br2 will displace I– ions.
NOTE : In general, a halogen of low atomic number will oxidise the halide ion of higher atomic number.
Q.6. The H-N-H bond angle in NH3 is greater than the H-As-H bond angle is AsH3. (1984 - 1 Mark)
Ans. T
Solution.
True : NOTE : The central element in the metal hydrides of group 15 elements is although in sp3 hybrid state, the H – M – H bond angle is less than the normal tetrahedral bond angle of 109º 28’; e.g. the bond angle, H – N – H in NH3 is 106º 45’.
This is due to greater repulsion between a lone pair and a bond pair of electrons than between the two bond pairs of electrons.
The decrease in bond angle from 106º 45’ in ammonia to about 90º in AsH3 can be explained by the fact that in the latter case sp3 hybridisation becomes less and less distinct with the increasing size of their electron clouds, i.e., pure p orbitals (instaed of sp3 hybrid orbitals) are used for M – H bonding and the lone pair of electrons is present in spherical s-orbital.
Q.7. Carbon tetrachloride is inflammable. (1985 - ½ Mark)
Ans. F
Solution. False : because of its high thermal stability. CCl4 is most stable as compared to other tetrachlorides of the group.
Q.8. Graphite is better lubricant on the moon than on the earth. (1987 - 1 Mark)
Ans. T
Solution. True : Graphite is better lubricant on the moon than on the earth because of lack of gravitation pull on the moon, where friction is already less than earth.
Q.9. All the Al–Cl bonds in Al2Cl6 are equivalent. (1989 - 1 Mark)
Ans. F
Solution.
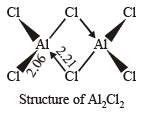
Bond distance between aluminium-chlorine bond forming bridge is greater (2.21 Å) than the distance between aluminium-chlorine bond present in the end (2.06 Å).
Q.10. Nitric oxide, though an odd electron molecule, is diamagnetic in liquid state. (1991 - 1 Mark)
Ans. T
Solution. True : The molecule of NO has eleven valence electrons (5 due to N and 6 due to O). It is impossible for all of them to be paired, hence the nitric oxide molecule contains an odd electron which makes gaseous nitric oxide paramagnetic.
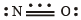
NOTE : In the liquid and solid states, nitric oxide is polymerised to a dimer which is diamagnetic.
Q.11. Diamond is harder than graphite. (1993 - 1 Mark)
Ans. T
Solution. True : In diamond, each carbon atom is in sp3 hybridised state and is linked to four other neighbouring carbon atoms held at the corners of a regular tetrahedron by covalent bonds. Owing to very strong covalent bonds by which the atoms are held together, diamond is the hardest substance known. Graphite has a two dimensional sheet like structure and carbon in sp2 hybridised state is attached to three other carbon atoms by three s bonds forming a hexagonal planar structure. Due to wide separation and weak interlayer bonds, the two adjacent layers can easily slide over each other; hence graphite is soft.
Q.12. The tendency for catenation is much higher for C than for Si. (1993 - 1 Mark)
Ans. T
Solution. True : The property of catenation in carbon is due to the fact that in carbon atom, the number of valence electrons (4) is equal to the number of valence orbitals (one 2s + three 2p). Hence carbon in the tetravalent state is fully saturated, i.e., it has neither any vacant orbital nor any lone pair of electrons on its atom due to which the C – C bond is extremely stable.
NOTE : The reason for greater tendency of carbon for catenation than silicon may further be explained by the fact that the C – C bond energy is approximately of the same magnitude as the energies of the bond between C and other elements. On the other hand, the Si – Si bond is weaker than the bonds between silicon and other elements.
Q.13. HBr is a stronger acid than HI because of hydrogen bonding. (1993 - 1 Mark)
Ans. F
Solution. False : None amongst, HBr and HI, HI exhibit hydrogen bonding. HI is a stronger acid than HBr because of its higher dissociation constant, Ka. HI has a stronger tendency to release protons to water molecules and hence is a stronger acid.
|
446 docs|929 tests
|





















