JEE Advanced Previous Year Questions (2018 - 2024): Alcohols, Phenols and Ethers | Chemistry for JEE Main & Advanced PDF Download
2024
Q1: Reaction of iso-propylbenzene with O₂ followed by the treatment with H₃O⁺ forms phenol and a by-product P. Reaction of P with 3 equivalents of Cl₂ gives compound Q. Treatment of Q with Ca(OH)₂ produces compound R and calcium salt S.The correct statement(s) regarding P, Q, R, and S is(are):
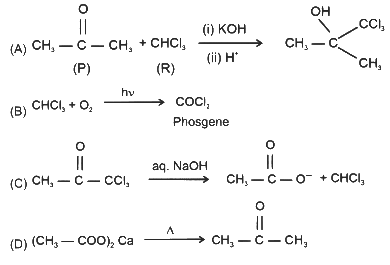
(a) Reaction of P with R in the presence of KOH followed by acidification gives
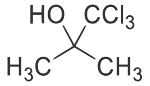
(b) Reaction of R with O₂ in the presence of light gives phosgene gas
(c) Q reacts with aqueous NaOH to produce CCl₃CCH₂OH and CCl₃CCOONa
(d) S on heating gives P [JEE Advanced 2024 Paper 1]
Ans: (a), (b), (d)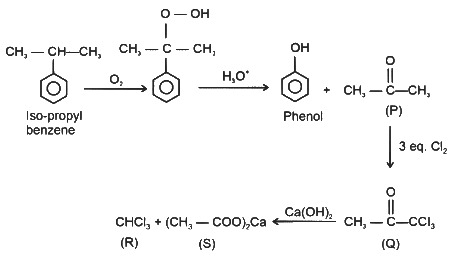
2023
Q1: In the given reaction scheme, P is a phenyl alkyl ether, Q is an aromatic compound; R and S are the major products.
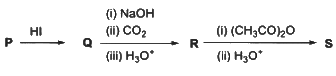
The correct statement about S is :
(a) It primarily inhibits noradrenaline degrading enzymes.
(b) It inhibits the synthesis of prostaglandin.
(c) It is a narcotic drug.
(d) It is ortho-acetylbenzoic acid. [JEE Advanced 2023 Paper 1]
Ans: (b) 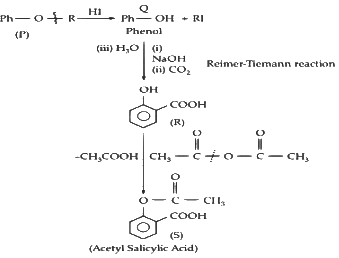
It inhibit synthesis of noradrenaline degrading enzymes.
Q2: Consider the following reaction scheme and choose the correct option(s) for the major products Q, R and S. 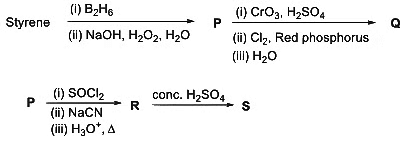
(a) 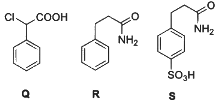
(b) 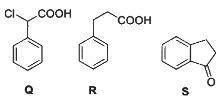
(c) 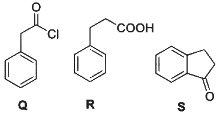
(d) 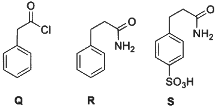 [JEE Advanced 2023 Paper 1]
[JEE Advanced 2023 Paper 1]
Ans: (b)
2020
Q1: An organic compound (C8H10O2) rotates plane-porarised light. It produces pink color with neutral FeCl3 solution. What is the total number of all the possible isomers for this compound? [JEE Advanced 2020 Paper 2]
Ans: 6
Phenolic (−OH) group gives positive test with neutral FeCl3 solution, means organic compound (C8H10O2) also rotate plane porarised light means compound is an optically active and chiral carbon atom is present.
So, the possible structures which are optically active and have phenolic group are as followed :
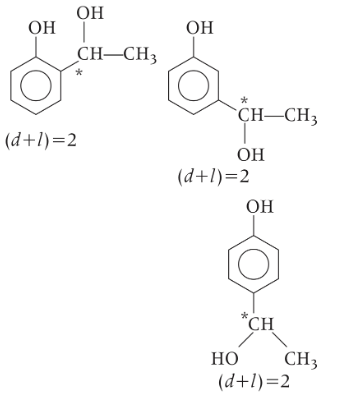
Therefore, total optically active isomers will be 6.
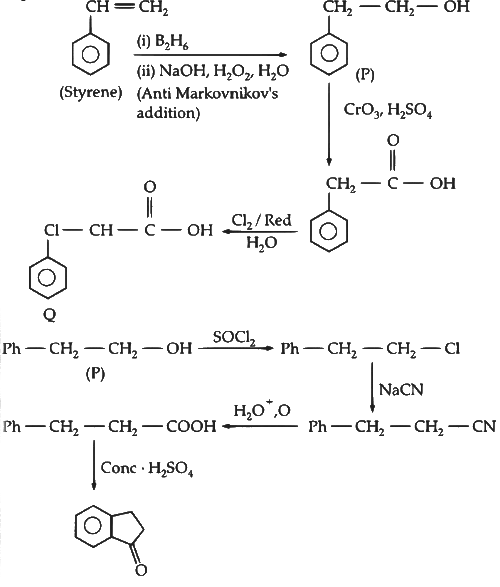
Q2: In the reaction scheme shown below Q, R and S are the major products.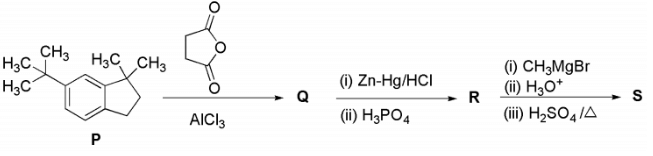
The correct structure of
(a) S is 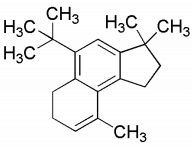
(b) Q is 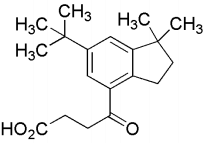
(c) R is 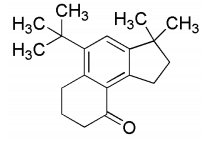
(d) S is 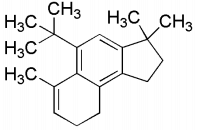 [JEE Advanced 2020 Paper 1]
[JEE Advanced 2020 Paper 1]
Ans: (b) & (d)
Compound (P) undergoes Friedel-Craft acylation reaction in presence of succinic anhydride and AlCl3 to gives an acid (Q).
Compound (Q) on treatment with Zn-Hg/HCl undergoes Clemmensen reduction, where ketone group change into alkane and on further reaction with dehydrating agent H3PO4 gives compound (R).
Compound (R) on reaction with Grignard reagent and heated in presence of acid gives dehydrating product (S).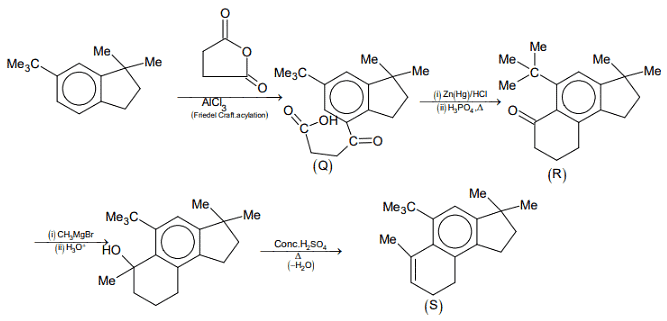
Thus, the option (b) and (d) are correct.
2018
Q1: LIST-I contains reactions and LIST-II contains major products.
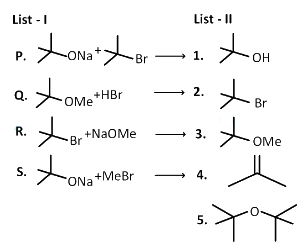
Ans: (b)
P. Reactant 1 , acts as a base and abstracts hydrogen from β carbon of tert butyl bromide halide (reactant 2 ) forming an alkene and an alcohol.
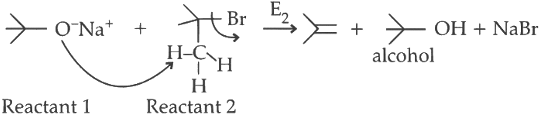 Elimination proceeds via E2 mechanism. Substitution of reactant 1 is not possible on reactant 2 due to steric crowding at 2.
Elimination proceeds via E2 mechanism. Substitution of reactant 1 is not possible on reactant 2 due to steric crowding at 2.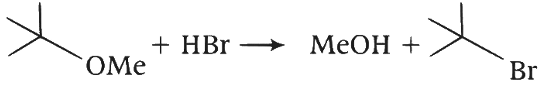 Q. Reactant 1 (tert butoxide) acts as a base and abstracts proton from acid (HBr) forming an alcohol and methyl bromide.
Q. Reactant 1 (tert butoxide) acts as a base and abstracts proton from acid (HBr) forming an alcohol and methyl bromide.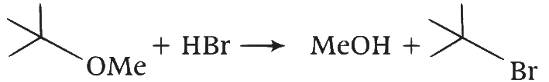
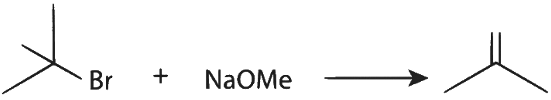

|
334 videos|660 docs|300 tests
|
FAQs on JEE Advanced Previous Year Questions (2018 - 2024): Alcohols, Phenols and Ethers - Chemistry for JEE Main & Advanced
| 1. What are the key differences between alcohols, phenols, and ethers? |  |
| 2. How do you differentiate between primary, secondary, and tertiary alcohols? |  |
| 3. What are the common methods for synthesizing alcohols? |  |
| 4. What is the significance of phenols in organic chemistry? |  |
| 5. How do ethers react with acids and bases? |  |
















