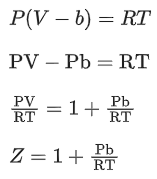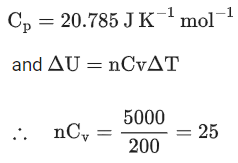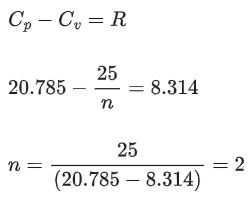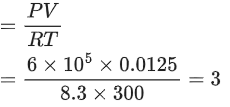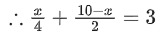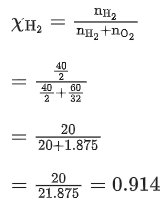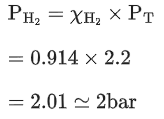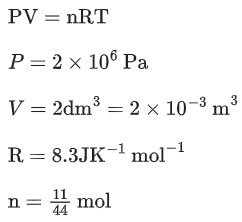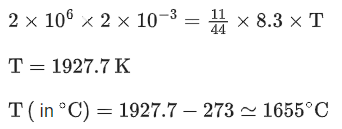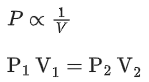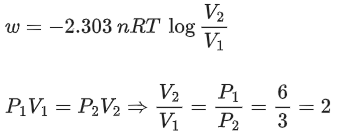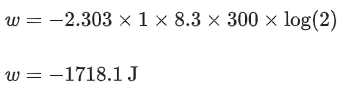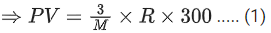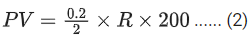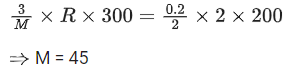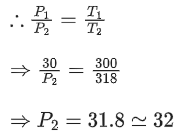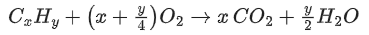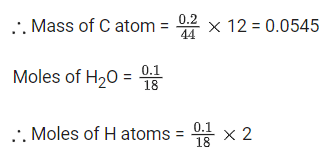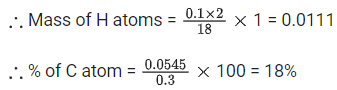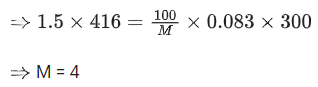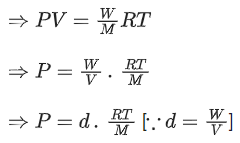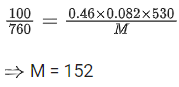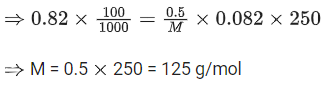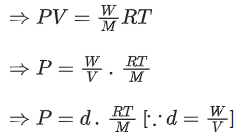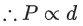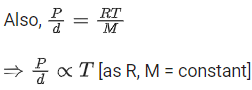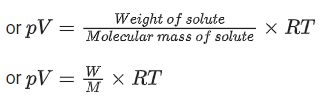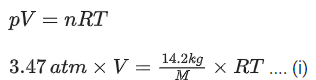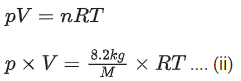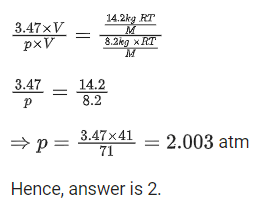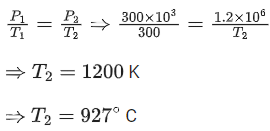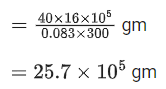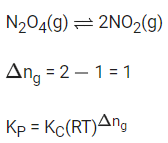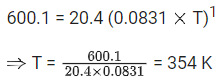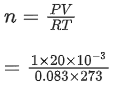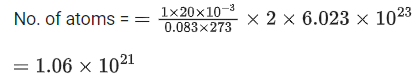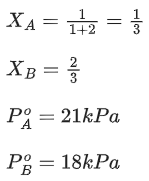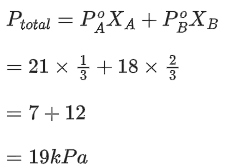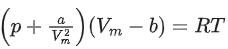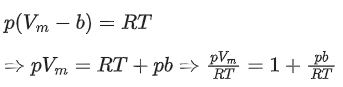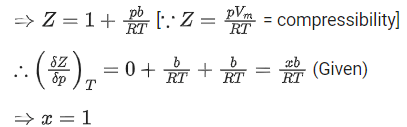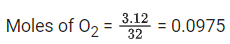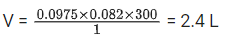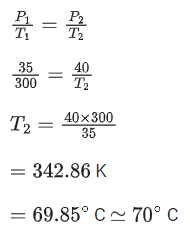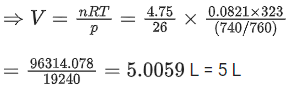JEE Main Previous year questions (2021-2025): Gaseous State | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Q.1. For a real gas at 25°C temperature and high pressure (99 bar) the value of compressibility factor is 2, so the value of Vander Waal's constant 'b' should be __________ ×10−2 L mol−1 (Nearest integer) (JEE Main 2022)
(Given R=0.083 L bar K−1 mol−1)
Ans. 25
For 1 mole at high pressure
Q.2. The molar heat capacity for an ideal gas at constant pressure is 20.785 J K−1 mol−1. The change in internal energy is 5000 J upon heating it from 300 K to 500 K. The number of moles of the gas at constant volume is ____________. [Nearest integer] (Given: R=8.314 J K−1 mol−1) (JEE Main 2022)
Ans. 2
and we know that
Q.3. A 10 g mixture of hydrogen and helium is contained in a vessel of capacity 0.0125 m3 at 6 bar and 27°C. The mass of helium in the mixture is ____________ g. (nearest integer)
Given: R=8.3 J K−1 mol−1
(Atomic masses of H and He are 1u and 4u, respectively) (JEE Main 2022)
Ans. 8
Number of moles of mixture of H2 and HeLet the mass of He in 10g mixture be xg
On solving x = 8g
∴ Mass of He in the mixture = 8g
Q.4. A mixture of hydrogen and oxygen contains 40% hydrogen by mass when the pressure is 2.2 bar. The partial pressure of hydrogen is bar. (Nearest Integer) (JEE Main 2022)
Ans. 2
40%w/w hydrogen gas is given in mixture of H2 and oxygen.Wt. of H2=40 g
Wt. of O2=60 g
Q.5. A sealed flask with a capacity of 2 dm3 contains 11 g of propane gas. The flask is so weak that it will burst if the pressure becomes 2 MPa. The minimum temperature at which the flask will burst is ___________ °C. [Nearest integer]
(Given : R = 8.3 J K-1 mol-1 , Atomic masses of C and H are 12u and 1u, respectively.) (Assume that propane behaves as an ideal gas.) (JEE Main 2022)
Ans. 1655
From ideal gas equation,
Q.6. The pressure of a moist gas at 27°C is 4 atm. The volume of the container is doubled at the same temperature. The new pressure of the moist gas is ________________ ×10−1 atm. (Nearest integer)
(Given : The vapour pressure of water at 27°C is 0.4 atm. ) (JEE Main 2022)
Ans. 22
From ideal gas equation,
Pressure of the gas =4−0.4=3.6 atm
3.6 V1=P2(2 V1)
P2=1.8 atm
Hence, new pressure of moist gas is 1.8+0.4=2.2 atm=22×10−1 atm
Q.7. 1.0 mol of monoatomic ideal gas is expanded from state 1 to state 2 as shown in the figure. The magnitude of the work done for the expansion of gas from state 1 to state 2 at 300 K is ____________ J. (Nearest integer)
(Given : R = 8.3 J K-1 mol-1, ln10 = 2.3, log2 = 0.30) (JEE Main 2022)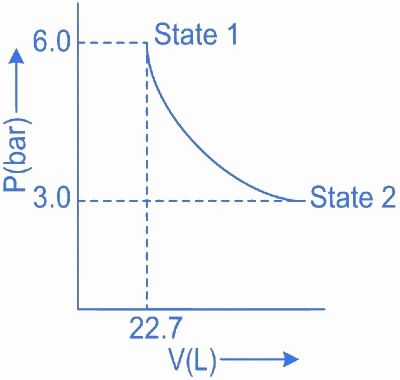
Ans. 1718
Q.8. At 300 K, a sample of 3.0 g of gas A occupies the same volume as 0.2 g of hydrogen at 200 K at the same pressure. The molar mass of gas A is ____________ g mol-1. (nearest integer) Assume that the behaviour of gases as ideal.
(Given : The molar mass of hydrogen (H2) gas is 2.0 g mol-1.) (JEE Main 2022)
Ans. 45
Both gas A and Hydrogen (H2) gas have same volume at same pressure. Let both 's volume is V and pressure P.
For gas A :
Pressure = P
Temperature (T) = 300 K
Volume = V
Mass = 3 g
Molar mass = M gm/mol
using ideal gas equation,
PV = nRT
For Hydrogen,
Pressure = P
Temperature (T) = 200 K
Volume = V
Mass = 0.2 g
Molar mass = 2 gm/mol
Using ideal gas equation,
From (1) and (2), we get
Q.9. A rigid nitrogen tank stored inside a laboratory has a pressure of 30 atm at 06:00 am when the temperature is 27°C. At 03:00 pm, when the temperature is 45°, the pressure in the tank will be _________ atm. [nearest integer] (JEE Main 2022)
Ans. 32
A nitrogen tank of fixed volume used where number of moles of nitrogen is fixed.
∴ V = constantn = constant
R = constant
From ideal gas equation,
PV = nRT
⇒ P ∝ T [As V, n, R = constant]
Here, initially P1 = 30 atm, T1 = 300 K
Finally, P2 = ?, T2 = 318 K
Q.10. On complete combustion 0.30 g of an organic compound gave 0.20 g of carbon dioxide and 0.10 g of water. The percentage of carbon in the given organic compound is _____________. (Nearest integer) (JEE Main 2022)
Ans. 18
Given organic compound CxHy= 0.3 gm
Produced carbon dioxide (CO2) = 0.2 gm
Produced water (H2O) = 0.1 gm
Moles of CO2 = 0.2/44
∴ Moles of C atom = 0.2/44
Q.11. 100 g of an ideal gas is kept in a cylinder of 416 L volume at 27°C under 1.5 bar pressure. The molar mass of the gas is __________ g mol-1. (Nearest integer)
(Given : R = 0.083 L bar K-1 mol-1) (JEE Main 2022)
Ans. 4
Given, Mass of ideal gas = 100 gm
Let the molar mass of ideal gas = M
∴ Number of moles of gas (n) = 100/M
Volume of cylinder (V) = 416 L
Temperature (T) = (27 + 273)K = 300 K
Pressure (P) = 1.5 bar
R = 0.083 L bar K−1 mol−1
Using ideal gas equation,
PV = nRT
Q.12. Geraniol, a volatile organic compound, is a component of rose oil. The density of the vapour is 0.46 gL-1 at 257°C and 100 mm Hg. The molar mass of geraniol is ____________ g mol-1. (Nearest Integer)
[Given : R = 0.082 L atm K-1 mol-1] (JEE Main 2022)
Ans. 152
From ideal gas equation we know
PV = nRT
We know, 760 mm of Hg = 1 atm
100 mm of Hg = 100/760 atm
Pressure (P) = 100/760 atm
Density (d) = 0.46
R = 0.082 L atm K-1 mol-1
T = (257 + 273) K = 530 K
Putting the values in above equation, we get
Q.13. An evacuated glass vessel weighs 40.0 g when empty, 135.0 g when filled with a liquid of density 0.95 g mL-1 and 40.5 g when filled with an ideal gas at 0.82 atm at 250 K. The molar mass of the gas in g mol-1 is :
(Given : R = 0.082 L atm K-1 mol-1) (JEE Main 2022)
(a) 35
(b) 50
(c) 75
(d) 125
Ans. d
Weight of empty glass vessel = 40 gm
Weight of glass vessel filled with liquid = 135 gm∴ Weight of liquid = 135 − 40 = 95 gm
Given density of liquid = 0.95 gm ml−1
∴ Volume of liquid =95/0.95=100 ml
Weight of glass filled with ideal gas = 40.5 gm
∴ Weight of gas = 40.5 − 40 = 0.5 g
Let the Molar mass = M
∴ Moles of gas =0.5/M
∴ Now applying ideal gas equation,
pV = nRT
Q.14. Which amongst the given plots is the correct plot for pressure (p) vs density (d) for an ideal gas? (JEE Main 2022)
(a) 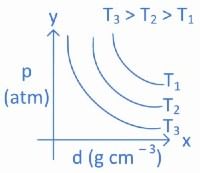
(b) 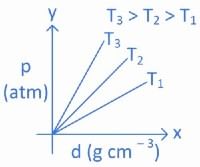
(c) 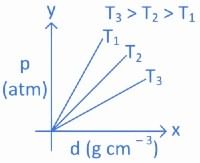
(d) 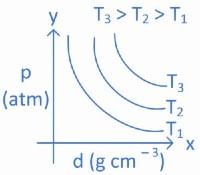
Ans. b
From ideal gas equation we know,
PV = nRT
For a fixed amount of gas at a fixed temperature, M and T is constant.
So graph between pressure (P) and density (d) is a straight line.
∴ When temperature T increases then slope of graph between P and d increases.
As T3 > T2 > T1 then slope of T3 will be highest then T2 and then T1.
So, graph (B) is the right graph.
Q.15. An empty LPG cylinder weighs 14.8 kg. When full, it weighs 29.0 kg and shows a pressure of 3.47 atm. In the course of use at ambient temperature, the mass of the cylinder is reduced to 23.0 kg. The final pressure inside of the cylinder is _________ atm. (Nearest integer)
(Assume LPG of be an ideal gas) (JEE Main 2021)
And. 2
Weight of empty LPG cylinder = 14.8 kg
Weight of full LPG cylinder = 29 kg
Weight of gas = 29 14.8 = 14.2 kg
If weight of full LPG cylinder = 23 kg
then weight of gas used = 29 23 = 6 kg at ambient temperature.
From ideal gas equation, pV = nRT
Applying ideal gas to LPG cylinder when gas is full,
Applying ideal gas to LPG cylinder when gas is reduced to 23 kg at ambient temperture,
Divide Eq. (i) by (ii)
Q.16. An LPG cylinder contains gas at a pressure of 300 kPa at 27°. The cylinder can withstand the pressure of 1.2 x 106 Pa. The room in which the cylinder is kept catches fire. The minimum temperature at which the bursting of cylinder will take place is __________°C. (Nearest integer) (JEE Main 2021)
Ans. 927
Q.17. A home owner uses 4.00x 103 m3 of methane (CH4) gas, (assume CH4 is an ideal gas) in a year to heat his home. Under the pressure of 1.0 atm and 300 K, mass of gas used is x *105 g. The value of x is __________. (Nearest integer)
(Given R = 0.083 L atm K-1 mol-1) (JEE Main 2021)
Ans. 26
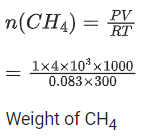
Q.18. The vapour pressures of A and B at 25°C are 90 mm Hg and 15 mm Hg respectively. If A and B are mixed such that the mole fraction of A in the mixture is 0.6, then the mole fraction of B in the vapour phase is x *10-1. The value of x is ___________. (Nearest integer) (JEE Main 2021)
Ans. 1
xA = 0.6PT
= xAPAo + xBPBo
= 0.6 × 90 + 0.4 × 15
= 54 + 6 = 60
xAPAo = yAPT
0.6 × 90 = yA(60)
⇒ yA = 0.9
yB = 0.1 = 1 × 10−1
∴ x = 1
Q.19. Consider the reaction
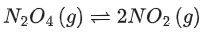
The temperature at which KC = 20.4 and KP = 600.1, is ____________ K. (Round off to the Nearest Integer). [Assume all gases are ideal and R = 0.0831 L bar K-1 mol-1] (JEE Main 2021)
Ans. 354
Q.20. The number of chlorine atoms in 20 mL of chlorine gas at STP is _________ 1021. (Round off to the Nearest Integer).
[Assume chlorine is an ideal gas at STP
R = 0.083 L bar mol-1 K-1, NA = 6.023 x 1023] (JEE Main 2021)
Ans. 1
Q.21. At 363 K, the vapour pressure of A is 21 kPa and that of B is 18 kPa. One mole of A and 2 moles of B are mixed. Assuming that this solution is ideal, the vapour pressure of the mixture is ___________ kPa. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 19
Q.22. A certain gas obeys P(Vm b) = RT. The value of 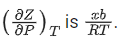 The value of x is _________. (Integer answer) (Z : compressibility factor) (JEE Main 2021)
The value of x is _________. (Integer answer) (Z : compressibility factor) (JEE Main 2021)
Ans. 1
For 1 mole of a real gas, the van der Waals' equation is,At very high pressure, the equation becomes,
Q.23. 3.12 g of oxygen is adsorbed on 1.2 g of platinum metal. The volume of oxygen adsorbed per gram of the adsorbent at 1 atm and 300 K in L is __________.
[R = 0.0821 L atm K-1mol-1] (JEE Main 2021)
Ans. 2
Using ideal gas equation : PV = nRT
∴ Volume of O2(g) adsorbed per gram of the adsorbent = 2.4/1.2 = 2
Q.24. A car tyre is filled with nitrogen gas at 35 psi at 27°C. It will burst if pressure exceeds 40 psi. The temperature in °C at which the car tyre will burst is _________. (Rounded off to the nearest integer) (JEE Main 2021)
Ans. 70
Q.25. The volume occupied by 4.75 g of acetylene gas at 50C and 740 mmHg pressure is __________ L. (Rounded off to the nearest integer)
[Given R = 0.0826 L atm K-1 mol-1] (JEE Main 2021)
Ans. 5
Given, mass of C2H2(g) = 4.75 g
Molecular weight = 26 g/mol
Temperature = 50 + 273 = 323 K
Pressure = 740 torr/mm of Hg
Pressure = 740/760 atm
R = 0.0821 L atm mol-1 K-1
Hence, no. of mole n = 4.75/26 mol
Formula used, pV = nRT (ideal gas)
|
347 docs|185 tests
|