Lakhmir Singh Manjit Kaur Solutions Class 10 Chemistry - Periodic Classification Of Elements- 2
(Page No - 283)
Question 20:
(a) Name three elements that have a single electron in their outermost shells.
(b) Name two elements that have two electrons in their outermost shells.
(c) Name three elements with completely filled outermost shells.
Solution :
(a) Lithium, Sodium, Potassium .
(b) Magnesium, Calcium .
(c) Helium, Neon, Argon .
Question 21:
What is Debereiner’s law of triads ? Explain with the help of one example of a Dobereiner’s triad.
Solution :
Dobereiner’s law of triads: When elements are arranged in order of increasing atomic masses, groups of three elements (triads), having similar chemical properties are obtained. The atomic mass of the middle elements of the triad being equal to the arithmetic mean of the atomic masses of the other two elements.
For example: Alkali metal group ( Dobereiner’s triad) : Lithium is the 1st element, sodium is the middle element whereas potassium is the 3rd element of the triad.
Question 22:
What is Newlands’ law of octaves ? Explain with an example.
Solution :
According to the Newlands’ law of octaves, when elements are arranged in the order of increasing atomic masses, the properties of the eighth element (starting from a given element) are a repetition of the properties of the first element.
For example: If we start with lithium as the first element, we find that the eighth element from it is sodium having the similar properties to lithium.
Question 23:
(a) Did Dobereiner’s triads also exist in the columns of Newlands’ law of octaves ? Explain your answer.
(b) What were the limitations of Dobereiner’s classification of elements ?
(c) What were the limitations of Newlands’ law of octaves ?
Solution :
(a)Yes,Dobereiners triads also exist in the columns of Newlands’ Octaves.
Consider the elements lithium (Li), sodium (Na) and potassiu m (K) which are present in the second column of Newlands’ classification of elements. Now, if we start with lithium as the 1 st element, then the 8 th element from it is sodium, and according to Newlands’ law of octaves, the properties of 8 th element , sodium should be similar to thos e of the 1 st element, lithium. Again, if we take sodium as the 1 st el ement , then the 8 th element from it is potassium, and according to Newlands ‘ law of octaves, the properties of 8 th element, potassium should be similar to those of the 1 st element, sodium. This means that according to Newlands’ law of octaves, the elements lithium, sodium and potassium should have similar chemical properties. We also know that lithium, sodium and potassium form a Dobereiner’s triad having similar chemical properties. From this, we conclude that Dobereiners triads also exist in the columns of Newlands Octaves.
(b) The main limitation of Dobereiner’s classification of elements was that it failed to arrange all the then known elements in the form of triads of elements having similar chemical properties. Dobereiner could identify only three triads from the elements known at that time. So, his classification of elements was not much successful. Another limitation was that Dobereiner failed to explain the relation between atomic masses of elements and their chemical properties.
(c) Newlands’ law of octaves for the classification of elements had the following limitations:
( i ) Newlands’ law of octaves was applicable to the classification of elements up to calcium only. After calcium, every eighth element did not possess the properties similar to that of the first element. Thus, this law worked well with lighter elements only.
(ii) Newlands assumed that only 56 elements existed in nature and no more elements would be discovered in the future. But later on, several new elements were discovered whose properties did not fit into Newlands’ law of octaves.
(iii) In order to fit elements into his table, Newlands put even two elements together in one slot and that too in the column of unlike elements having very different properties. For example, the two elements cobalt (Co) and nickel (Ni) were put together in just one slot and that too in the column of elements like fluorine, chlorine and bromine which have very different properties from these elements.
Question 24:
(a) State the periodic law on which Mendeleev’s periodic table was based. Why and how was this periodic law changed ?
(b) Explain why, the noble gases are placed in a separate group.
Solution :
(a) According to Mendeleev’s periodic law: The properties of elements are a periodic function of their atomic masses. It was the discovery of atomic number which led to a change in Mendeleev’s periodic law which was based on atomic mass.
(b) The noble gases are placed in a separate group because they are chemically very inert or unreactive (having completely filled outermost electron shells).
Question 25:
(a) State the merits of Mendeleev’s classification of elements.
(b) Describe two anomalies of Mendeleev’s periodic classification of elements.
Solution :
(a) Merits of Mendeleev’s classification of elements:
(i) Mendeleev’s periodic law predicted the existence of some elements that had not been discovered at that time.
(ii) Mendeleev’s periodic table could predict the properties of several elements on the basis of their positions in the periodic table.
(iii) It could accommodate noble gases when they were discovered.
(b) Anomalies of Mendeleev’s classification of elements:
(i) The position of isotopes could not be explained: If the elements are arranged according to atomic masses, the isotopes should be placed in different groups of the periodic table. But, the isotopes were not given separate places in Mendeleev’s periodic table. They were placed at the same place in the table. This placing of the isotopes at same place could not be explained by Mendeleev’s periodic law.
(ii) Wrong order of atomic masses of some elements could not be explained: In Mendeleev’s periodic table, when certain elements were put in their correct group on the basis of their chemical properties, it was found that the element with higher atomic mass comes first and the element with lower atomic mass comes later. Mendeleev’s periodic law could not explain this abnormal situation of wrong order of atomic masses.
Question 26:
(a) How do the properties of eka-aluminium element predicted by Mendeleev compare with the actual properties of gallium element ? Explain your answer.
(b) What names were given by Mendeleev to the then undiscovered elements (i) scandium (ii) gallium, and (iii) germanium ?
Solution :
(a) Eka-aluminium and gallium are the two names of the same element as Eka -Aluminum has almost exactly the same properties as the actual properties of the gallium element. The properties: atomic mass, density, melting point, formula of chloride and formula of oxide are almost the same.
(b) (i) Eka boron.
(ii) Eka aluminum.
(iii) Eka -silicon.
Question 27:
(a) Why do we classify elements ?
(b) What were the two criteria used by Mendeleev to classify the elements in his periodic table ?
(c) Why did Mendeleev leave some gaps in his periodic table ?
(d) In Mendeleev’s periodic table, why was there no mention of noble gases like helium, neon and argon ?
(e) Would you place the two isotopes of chlorine, Cl-35 and Cl-37 in different slots because of their different atomic masses or in the same slot because their chemical properties are the same ? Justify your answer .
Solution :
(a) The elements are classified into groups so that the elements with similar properties fall in the same group and hence the study of a large number of elements is reduced to the study of a few group of elements.
(b)(i) Increasing atomic masses
(ii) Grouping together of elements having similar properties.
(c) In order to make sure that the elements having similar properties fell in the same vertical column or group, Mendeleev left some gaps in his periodic table.
(d) Out of eight groups in the original periodic table of Mendeleev, first seven groups are of normal elements and eighth group is of transition elements. Noble gases were not known at that time. So, there was no group of noble gases in Mendeleev’s table.
(e) The isotopes of chlorine, Cl-35 and Cl-37 are placed in the same slot because they have similar chemical properties and same atomic number.
Question 28:
(a) State Mendeleev’s periodic law.
(b) What chemical properties of elements were used by Mendeleev in creating his periodic table ?
(c) State any three limitations of Mendeleev’s classification of elements.
(d) Besides gallium, which two other elements have since been discovered for which Mendeleev had left gaps in his periodic table ?
(e) Which group of elements was missing from Mendeleev’s original periodic table ?
Solution :
(a) Mendeleev’s periodic law: The properties of elements are a periodic function of their atomic masses. It was the discovery of atomic number which led to a change in Mendeleev’s periodic law which was based on atomic mass.
(b) The elements having similar chemical properties form oxides and hydrides having similar formulae. Mendeleev used these properties for creating his periodic table.
(c) Limitations of Mendeleevs classification of elements:
(i) The position of isotopes could not be explained.
(ii) Wrong order of atomic masses of some elements could not be explained.
(iii) A correct position could not be assigned to Hydrogen in the periodic table.
(d) Silicon and Germanium.
(e) Noble gases were missing from Mendeleev’s original periodic table.
Question 29:
(a) State modern periodic law.
(b) How does the electronic configuration of the atom of an element relate to its position in the modern periodic table ?
(c) How could the modern periodic law remove various anomalies of Mendeleev’s periodic table ? Explain with examples.
(d) Is it possible to have an element having atomic number 1.5 placed between hydrogen and helium ?
(e) Name the scientist who prepared modern periodic table.
Solution :
(a) The modern periodic law states that the properties of elements are a periodic fu nction of their atomic numbers.
(b) When elements are arranged according to increasing atomic numbers, there is a periodicity in the electronic configurations of the elements. The elements in a period have consecutive atomic numbers. The elements having same number of valence electrons in their atoms are placed in a group. All the elements in a group have similar electronic configurations and show similar properties.
(c) When the elements are arranged according to their atomic numbers on the basis of modern periodic law, then all the anomalies (or defects) of Mendeleev’s classification disappear. This is discussed below:
(i) Explanation for the Position of Isotopes: All the isotopes of an element have the same number of protons, so their atomic number is also the same. Since all the isotopes of an element have the same atomic number, they can be put at one place in the same group of the periodic table. For example, both the isotopes of chlorine, Cl-35 and Cl-37, have the same atomic number of 17, so both of them can be put at one place in the same group of the periodic table.
(ii) Explanation for the Position of Cobalt and Nickel: The atomic number of cobalt is 27 and that of nickel is 28. Now, according to modern periodic law, the elements are arranged in the order of increasing atomic numbers. So, cobalt with lower atomic number (27) should come first and nickel with higher atomic number (28) should come later, even if their atomic masses are in the wrong order.
(iii) Explanation for the Position of Hydrogen: Hydrogen element has been placed at the top of group 1, above the alkali metals because the electronic configuration of hydrogen is similar to those of alkali metals. Both, hydrogen as well as alkali metals have 1 valence electron each.
(d) Atomic number is always a simple whole number. It can either be 1 or 2. There can be no element with atomic number 1.5.
(e) The modern periodic table was prepared by Bohr .
(Page No - 284)
Question 42:
The atomic masses of three elements X, Y and Z having similar chemical properties are 7, 23 and 39 respectively.
(a) Calculate the average atomic mass of elements X and Z.
(b) How does the average atomic mass of elements X and Z compare with the atomic mass of element Y ?
(c) Which law of classification of elements is illustrated by this example ?
(d) What could the elements X, Y and Z be ?
(e) Give another example of a set of elements which can be classified according to this law.
Solution :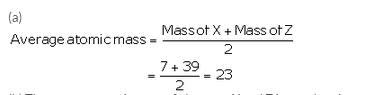
(b) The average atomic mass of elements X and Z is equal to the atomic mass of element Y
(c) Dobereiner’s law of triads.
(d) X is lithium, Y is sodium and Z is potassium .
(e) Chlorine, Bromine, Iodine .
Question 43:
In the following set of elements, one element does not belong to the set. Select this element and explain why it does not belong :
Calcium, Magnesium, Sodium, Beryllium
Solution :
Sodium does not belong to the set. This is because all other elements belong to group 2 but sodium belongs to group 1 .
Question 44:
In the following set of elements, one element does not belong to the set. Select this element and state why it does not belong :
Oxygen, Nitrogen, Carbon, Chlorine, Fluorine
Solution :
Chlorine does not belong to the set. This is because all other elements belong to 2nd period whereas chlorine belongs to 3rd period.
Question 45:
Can the following groups of elements be classified as Dobereiner’s triads ?
(a) Na, Si, Cl (b) Be, Mg, Ca
Give reason for your answer.
(Atomic masses : Be 9 ; Na 23 ; Mg 24 ; Si 28 ; Cl 35.5 ; Ca 40)
Solution :
(a) No. This is because the elements Na, Si and Cl do not have similar properties even though the atomic mass of middle element Si is almost equal to the average atomic mass of first element Na and third element Cl.
(b) Yes. This is because the elements Be, Mg and Ca have similar properties and the atomic mass of middle element Mg is almost equal to the average atomic mass of first element Be and third element Ca
Question 46:
Consider the following elements :
Na, Ca, Al, K, Mg, Li
(a) Which of these elements belong to the same period of the periodic table ?
(b) Which of these elements belong to the same group of the periodic table ?
Solution :
(a) Same period (Third period): Na, Mg, Al.
(b) Same group (First group): Li, Na, K .
Question 47:
Which element has :
(a) two shells, both of which are completely filled with electrons ?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells, with four electrons in its valence shell ?
(d) a total of two shells, with three electrons in its valence shell ?
(e) twice as many electrons in its second shell as its first shell ?
Solution :
(a) Neon (2, 8).
(b) Magnesium .
(c) Silicon (2, 8, 4).
(d) Boron (2, 3) .
(e) Carbon (2, 4)
(Page No - 285)
Question 48:
Consider the following elements :
Ca, Cl, Na, I, Li, Ba, Sr, K, Br
Separate these elements into three groups (families) of similar properties. State one property in each case on the basis of which you have made your choice.
Solution :
Li,Na,K : All these elements ar e metals having a valency of 1.
Ca,Sr,Ba: All these elements are metals having a valency of 2.
Cl,Br,I : All these elements are halogens.
Question 49:
Mendeleev predicted the existence of certain elements not known at that time and named two of them as eka-aluminium, and eka-silicon.
(a) Name the element which has taken the place of (i)eka-aluminium, and (ii)eka-silicon
(b) Mention the period/periods of these elements in the modern periodic table.
(c) Write the group/groups of these elements in the modern periodic table.
(d) Classify these elements as metals, non-metals or metalloids.
(e) How many valence electrons are present in the atoms of each of these elements ?
Solution :
(a) (i) Gallium (ii) Germanium.
(b) 4th period .
(c) Gallium: 13th group; Germanium: 14th group .
(d) Gallium: Metal; Germanium: Metalloid .
(e) Gallium: 3 ; Germanium: 4 .
Question 50:
A part of the early classification of elements has been given below :
H Li Be B C N O
F Na Mg A1 Si P S
(a) Which law of classification of elements is illustrated by the above arrangement of elements ?
(b) Name the scientist who proposed such a classification of elements.
(c) Why is such a classification of elements compared with a characteristic of musical scale ?
(d) State one limitation of this classification of elements.
Solution :
(a) Newlands’ law of octaves .
(b) Newlands .
(c) This classification of elements is compared with a characteristic of musical scale because in this classification, the repetition in the properties of elements is just like the repetition of eighth note in an octave of music .
(d) This classification of elements could be applied only up to the element calcium and not beyond that .
(Page No - 302)
Question 1:
Given alongside is a part of the periodic table :
As we move horizontally from left to right :
(i) What happens to the metallic character of the elements ?
(ii) What happens to the atomic size ?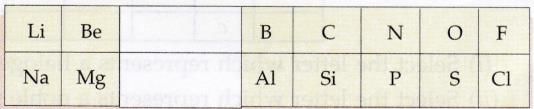
Solution :
(i) Metallic character decreases.
(ii) Atomic size decreases .
Question 2:
How would the tendency to gain electrons change on moving from left to right in a period of the periodic table ?
Solution :
On moving from left to right in a period, the tendency of atoms to gain electrons increases.
Question 3:
How would the tendency to lose electrons change as we go from left to right across a period of the periodic table ?
Solution :
On moving from left to right in a period, the tendency of atoms to lose electrons decreases
FAQs on Lakhmir Singh Manjit Kaur Solutions Class 10 Chemistry - Periodic Classification Of Elements- 2
| 1. What is the periodic classification of elements? |  |
| 2. How many periods are there in the periodic table? |  |
| 3. What is the significance of the periodic table? |  |
| 4. What are the groups in the periodic table? |  |
| 5. Why is the periodic table considered a tool for chemists? |  |
|
61 docs
|

|
Explore Courses for Class 10 exam
|

|

















