Class 9 Science Chapter 1 Question Answers - Matter in Our Surroundings
Q1: Discuss the factors which affect evaporation.
Ans: Evaporation is the process by which liquid changes into vapour at temperatures below its boiling point. Several factors influence the rate of evaporation. These include:
1. Surface Area:
The rate of evaporation increases with the increase in surface area. This is because more molecules are exposed to the surface and can escape into the atmosphere. For example, wet clothes dry faster when spread out.
2. Temperature:
When the temperature rises, the kinetic energy of the particles increases. This allows more molecules to overcome the force of attraction and escape from the liquid surface as vapour.
3. Wind Speed:
A higher wind speed removes the evaporated particles from the surface quickly, allowing more particles to escape. This increases the rate of evaporation.
4. Humidity:
Humidity refers to the amount of water vapour present in the air. If the air is already humid, it cannot take in more vapour, so the rate of evaporation decreases.
Thus, evaporation is faster when the surface area and temperature are high, the wind is strong, and the humidity is low.
Q2: Explain the interconversion of three states in terms of force of attraction and kinetic energy of the molecules.
Ans: Matter exists in three main states—solid, liquid, and gas. These states can change into one another by varying temperature and pressure. These changes are based on two key properties of particles:
- Intermolecular Force of Attraction
- Kinetic Energy
1. Solids:
In solids, the particles are tightly packed due to strong forces of attraction. The kinetic energy is very low, so particles only vibrate at fixed positions.
2. Liquids:
When a solid is heated, its particles gain energy and vibrate more vigorously. At a certain temperature (melting point), the force of attraction is weakened, and the solid becomes a liquid. Particles in liquids have more kinetic energy and can slide past each other.
3. Gases:
Further heating of the liquid increases kinetic energy so much that the particles overcome almost all the attraction and move freely. The liquid changes into gas (boiling point). Gases have the weakest forces of attraction and the highest kinetic energy.
Hence, increasing temperature or decreasing pressure leads to a change of state by altering the kinetic energy and intermolecular attraction among particles. Interconversion of three states
Interconversion of three states
Changes in temperature or pressure can cause these states to convert into one another:
- Sublimation: Solid to gas without becoming liquid.
- Deposition: Gas to solid without becoming liquid.
- Boiling: Liquid to gas throughout the liquid.
- Evaporation: Liquid to gas from the surface.
Q3: How is the high compressibility property of gas useful to us?
Ans: Gases have a unique property called compressibility, which means they can be compressed easily because the particles are far apart. This property is extremely useful in our daily life and in industrial applications.
Uses of Compressibility:
LPG (Liquefied Petroleum Gas):
LPG is a fuel made by compressing petroleum gas into a liquid and storing it in cylinders. When the pressure is released during use, it returns to gaseous form and is used for cooking.Oxygen Cylinders:
Oxygen used in hospitals is stored in compressed form in metal cylinders. This allows a large quantity of oxygen to be carried in a small container.CNG (Compressed Natural Gas):
CNG is methane gas compressed into high-pressure containers. It is used as a fuel for vehicles because it is cleaner and occupies less space in compressed form.
Thus, the compressibility of gases makes it possible to store and transport them efficiently for domestic, medical, and industrial use.
Q4: Pressure and temperature determine the state of a substance. Explain this in detail.
Ans: The state of any matter (solid, liquid, or gas) depends on its temperature and the pressure applied to it. Both of these factors affect the particle movement and the intermolecular forces of attraction.
Effect of Temperature:
When the temperature increases, particles gain kinetic energy and start moving faster.
For example, if ice (solid) is heated, it melts into water (liquid). On further heating, the water boils and becomes steam (gas).
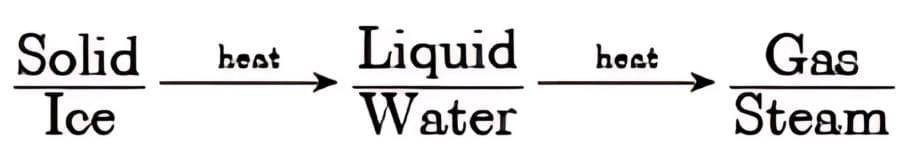
Similarly, cooling removes energy. Steam condenses into water, and upon further cooling, it forms ice.
Effect of Pressure:
An increase in pressure brings particles closer, which strengthens intermolecular forces and may change a gas into a liquid or a liquid into a solid

For instance, carbon dioxide gas changes into dry ice (solid) when high pressure is applied and the temperature is lowered.
In LPG cylinders, the gas is compressed under pressure to store it in liquid form. When the gas is used, the pressure is released and it becomes gaseous.
Both temperature and pressure play important roles in changing the state of a substance by affecting molecular motion and spacing between particles.
Q5: Give the difference between Evaporation and Boiling.
Ans: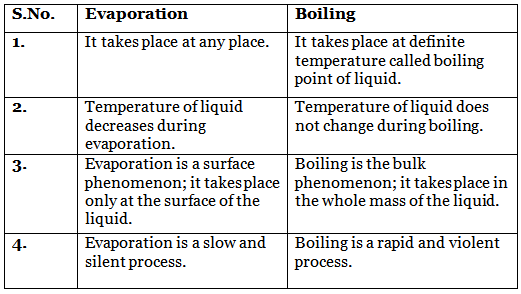
Q6: The melting point of ice is 273.15 K. What does this mean? Explain in detail.
Ans: The melting point of a solid is the temperature at which it changes into a liquid at normal atmospheric pressure. For ice, this melting point is 273.15 K (0°C).
Explanation:
- At 273.15 K, ice starts to melt into water.
- The temperature remains constant during this process, even though heat is continuously supplied.
- The heat energy is used to overcome the strong intermolecular forces holding the solid particles together.
- This energy is called the latent heat of fusion.
- Once all the ice melts, the temperature of the resulting liquid water starts to rise if more heat is supplied.
The melting point of ice signifies the temperature at which it turns into liquid water without any change in temperature, by absorbing latent heat.
Q7: With the help of an example, explain how the diffusion of gases in water is essential.
Ans: Diffusion is the process by which particles move from an area of higher concentration to an area of lower concentration. Gases from the atmosphere, such as oxygen and carbon dioxide, diffuse into water, and this diffusion is vital for aquatic life.
Importance of Diffusion in Water:
- Oxygen from the air dissolves in water through diffusion. Aquatic animals like fish absorb this oxygen through their gills for respiration.
- Carbon Dioxide also diffuses into water and is used by aquatic plants during photosynthesis to make food.
Example:
Fish survive in water because they take in the dissolved oxygen, which has diffused from the air into the water. Similarly, underwater plants use dissolved carbon dioxide. Thus, the diffusion of gases like oxygen and carbon dioxide in water is crucial for the survival of aquatic organisms.
|
85 videos|549 docs|60 tests
|
FAQs on Class 9 Science Chapter 1 Question Answers - Matter in Our Surroundings
| 1. What is matter, and how is it classified? |  |
| 2. What are the three states of matter, and how do they differ from each other? |  |
| 3. How does temperature affect the state of matter? |  |
| 4. What are the physical and chemical properties of matter? |  |
| 5. What is the difference between a homogeneous mixture and a heterogeneous mixture? |  |






















