JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: Thermodynamics
Mind Map: Thermodynamics | Chemistry for JEE Main & Advanced PDF Download
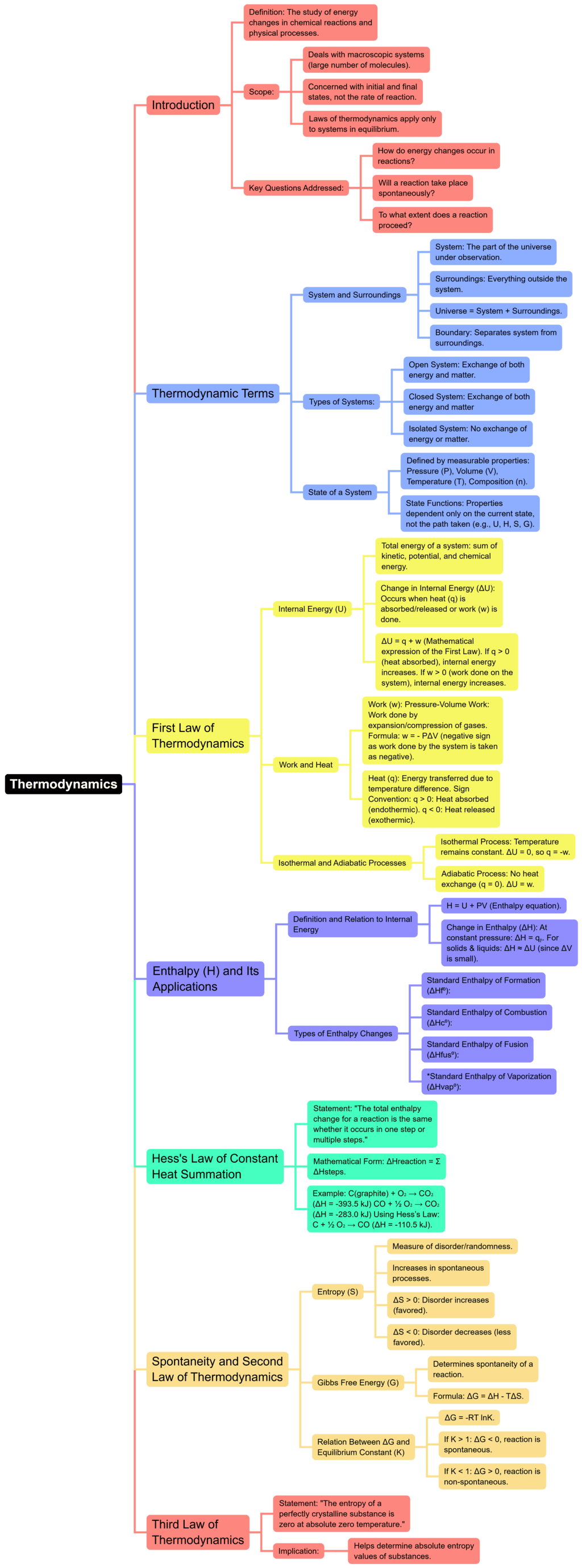
The document Mind Map: Thermodynamics | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
361 videos|822 docs|301 tests
|
FAQs on Mind Map: Thermodynamics - Chemistry for JEE Main & Advanced
| 1. What are the main laws of thermodynamics? |  |
Ans.The main laws of thermodynamics are four fundamental principles that describe how energy moves within physical systems. They are:
1. <b>Zeroth Law of Thermodynamics</b>: If two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
2. <b>First Law of Thermodynamics</b>: This law states that energy cannot be created or destroyed, only transformed from one form to another. This is often expressed as ΔU = Q - W, where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
3. <b>Second Law of Thermodynamics</b>: It states that the total entropy of an isolated system can never decrease over time. Heat cannot spontaneously flow from a colder body to a hotter body.
4. <b>Third Law of Thermodynamics</b>: As the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value.
| 2. What is the significance of the concept of entropy in thermodynamics? |  |
Ans.Entropy is a measure of the disorder or randomness in a system. It is significant because it helps to quantify the amount of energy in a system that is unavailable to do work. The second law of thermodynamics states that the entropy of an isolated system will always increase over time, leading to the conclusion that natural processes favor an increase in disorder. In practical terms, understanding entropy allows scientists and engineers to predict the direction of spontaneous processes and the efficiency of energy conversions.
| 3. How does the Carnot cycle relate to thermodynamics? |  |
Ans.The Carnot cycle is an idealized thermodynamic cycle that provides a standard of performance for heat engines. It consists of four reversible processes: two isothermal (constant temperature) and two adiabatic (no heat exchange). The significance of the Carnot cycle lies in its demonstration of the maximum possible efficiency that any heat engine can achieve, which is determined by the temperatures of the hot and cold reservoirs. The efficiency (η) of a Carnot engine is given by η = 1 - (Tₗ/Tₕ), where Tₗ and Tₕ are the absolute temperatures of the cold and hot reservoirs, respectively.
| 4. What is the difference between an isothermal process and an adiabatic process? |  |
Ans.An isothermal process occurs at a constant temperature, meaning that any heat added to the system is used to do work, while the internal energy remains constant. In contrast, an adiabatic process involves no heat transfer to or from the system, which means that any work done on or by the system results in a change in its internal energy. Isothermal processes typically occur in ideal gases at a constant temperature, while adiabatic processes can occur in various systems where insulation is present.
| 5. What are real-world applications of thermodynamics? |  |
Ans.Thermodynamics has numerous practical applications across various fields. In engineering, it is crucial for the design of engines, refrigerators, and heat pumps. In the chemical industry, thermodynamics helps in understanding reaction equilibria and optimizing processes. Environmental science uses thermodynamic principles to study energy transfers in ecosystems and the impact of energy consumption on climate. Additionally, thermodynamics is essential in fields like material science, where it aids in the development of new materials through the understanding of phase changes and stability.
Related Searches





















