JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: d and f Block Elements
Mind Map: d and f Block Elements | Chemistry for JEE Main & Advanced PDF Download
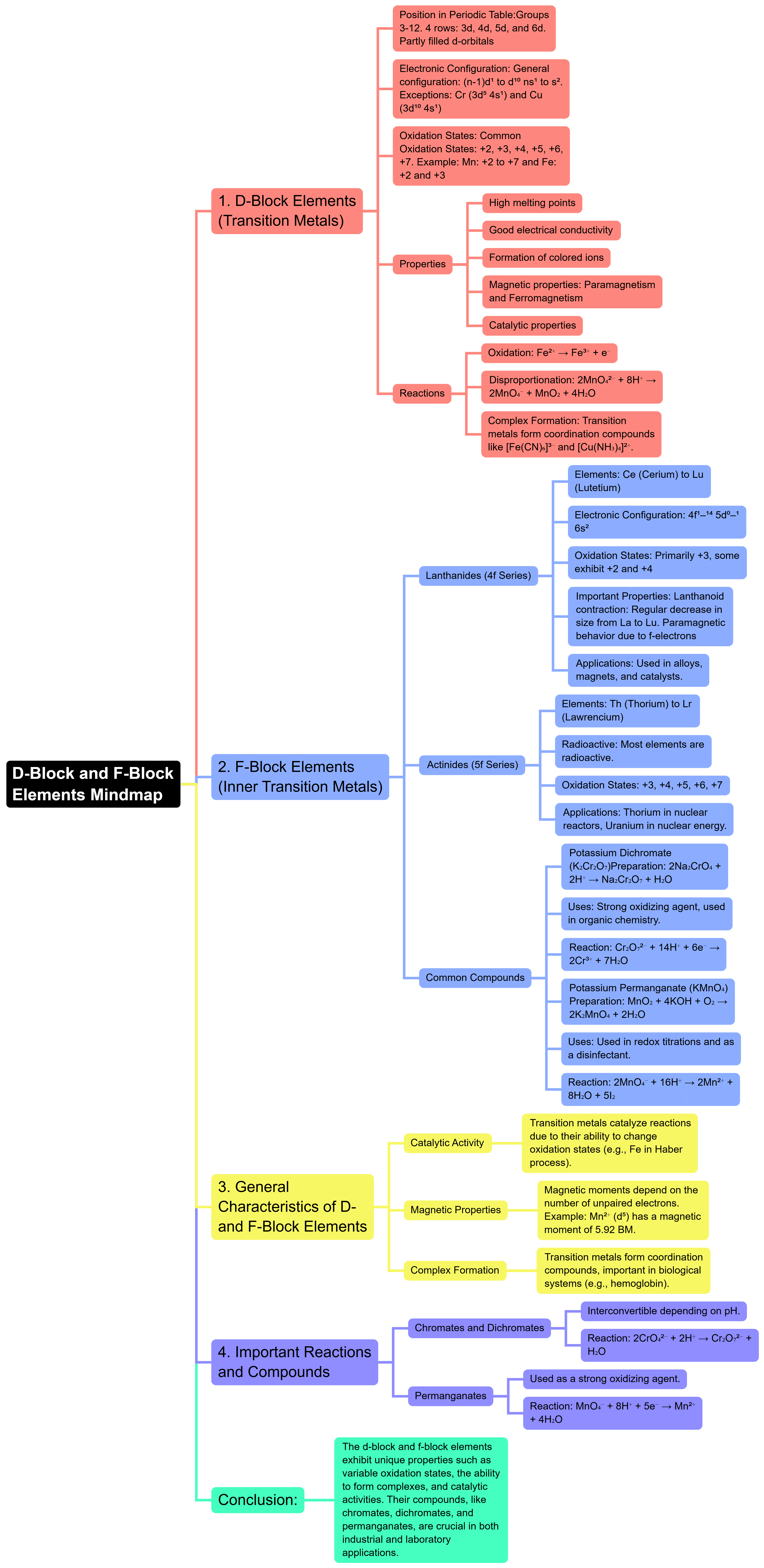
The document Mind Map: d and f Block Elements | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
361 videos|822 docs|301 tests
|
FAQs on Mind Map: d and f Block Elements - Chemistry for JEE Main & Advanced
| 1. What are d and f block elements in the periodic table? |  |
Ans. The d block elements, also known as transition metals, are found in groups 3 to 12 of the periodic table. They are characterized by the filling of d orbitals. These elements are known for their ability to form variable oxidation states and complex ions, as well as for their catalytic properties. The f block elements include the lanthanides and actinides, which are located at the bottom of the periodic table. They are characterized by the filling of f orbitals and are known for their unique magnetic and electronic properties.
| 2. What are the common properties of d block elements? |  |
Ans. Common properties of d block elements include high melting and boiling points, electrical conductivity, the ability to form colored compounds, and the presence of multiple oxidation states. They tend to have high density and are generally good conductors of heat and electricity. Many d block elements also exhibit paramagnetism due to the presence of unpaired electrons in their d orbitals.
| 3. How do the f block elements differ from the d block elements? |  |
Ans. The f block elements differ from the d block elements primarily in their electronic configuration and position in the periodic table. While d block elements fill their d orbitals, f block elements fill their f orbitals. Additionally, f block elements include lanthanides and actinides, which have distinct properties such as radioactivity in actinides and unique optical properties in lanthanides. F block elements typically have lower densities and melting points compared to d block elements.
| 4. What are some applications of d and f block elements in real life? |  |
Ans. D block elements have numerous applications in industries, including their use as catalysts in chemical reactions, in the manufacture of alloys, and in electronic devices due to their conductive properties. For example, iron (Fe) is used to make steel, while platinum (Pt) is used in catalytic converters. F block elements, particularly those in the lanthanide series, are used in the production of strong magnets, phosphors in lighting, and in nuclear reactors due to the properties of certain actinides like uranium (U) and plutonium (Pu).
| 5. Why are transition metals known for forming complex ions? |  |
Ans. Transition metals are known for forming complex ions due to their ability to adopt various oxidation states and their partially filled d orbitals, which allow them to bond with ligands through coordinate covalent bonds. This property enables transition metals to form stable complexes with a wide variety of ligands, including water, ammonia, and various anions. The ability to form these complexes is crucial in biological systems, industrial processes, and in the development of various materials.
Related Searches
















