Class 7 Science Chapter 7 NCERT Based Activity - Heat Transfer in Nature
Activity 7.1: Let us experiment
- Caution—This activity should be carried out under the supervision of a teacher or an adult.
- Take a strip of a metal, such as aluminium or iron, about 15 cm long.
- Attach four pins to the strip with the help of wax such that they are arranged at nearly equal distances (about 2 cm apart), as shown in Fig. 7.1.
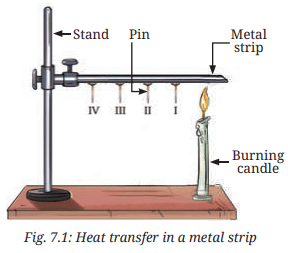
- Secure the strip to a stand and label the pins as I, II, III, and IV, as shown in Fig. 7.1. (If a stand is not available, place the strip between two bricks for support.)
- Heat the end of the strip that is away from the stand with a candle or a spirit lamp.

1. What will happen to the pins? Will they remain attached to the strip or will they fall?
Ans: The pins will fall from the strip as the wax holding them melts due to heat.
2. Predict the order in which the pins will fall from the strip:
Ans: Prediction: Pin I (closest to the heat source) will fall first, followed by Pin II, Pin III, and Pin IV.
3. Record your observations in Table 7.1:

4. Why does pin I fall before pin II? Why did all the pins not fall together?
Ans: Pin I falls before Pin II because it is closest to the heat source, so the wax holding it melts first as heat travels along the strip via conduction (page 3). The pins do not fall together because heat transfer takes time to reach each pin, with the wax melting sequentially as the heat progresses from the heated end (page 3: “Heat travels along the strip”).
5. From your observations, what can you infer?
Ans: Heat is transferred along the metal strip from the hot end to the colder end through conduction, where particles pass heat to their neighbors without moving from their positions (page 3).
Explanation: This states that metals are good conductors, allowing heat to pass easily (page 3: “Materials like metals… are good conductors”). The sequential falling of pins demonstrates conduction, as heat travels progressively along the strip, melting wax at each pin (page 3).
Activity 7.2: Let us investigate
- Take two identical paper cups.
- Hang them using threads of equal length in an inverted position on the two ends of a wooden stick, as shown in Fig. 7.3a.
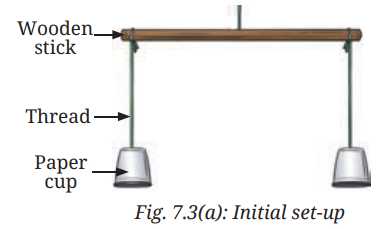
- Now, adjust the positions of the cups, so that the stick is horizontal.
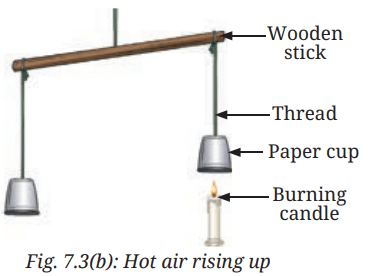
- Place a burning candle below one of the cups (Fig. 7.3b).
- Table 7.3: Recording observations and probable reasons
1. Observe what happens to the cup:
Ans: The cup under which the candle is placed rises upward (page 5).
2. Record your observations in Table 7.3 and think of probable reasons:
Explanation: This explains that heated air expands, becomes less dense, and rises, as seen with smoke or balloons (page 5: “Smoke… is warmer… rises up”). This is convection, where heat transfer occurs by the movement of particles (page 9). The rising cup demonstrates convection in air.
Activity 7.3: Let us find out
- Caution—This activity should be carried out under the supervision of a teacher or an adult.
- Take a 500 mL beaker, half-filled with water as shown in Fig. 7.5a.
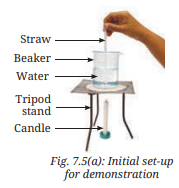
- With the help of a straw, place a grain of potassium permanganate at the centre of the beaker’s base (Fig. 7.5a).
- Place a candle right below the centre of the base of the beaker.
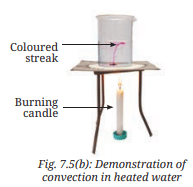
1. Observe what happens.
Ans: The potassium permanganate dissolves, and colored streaks rise upward in a circular pattern, indicating water movement (page 6, Fig. 7.5b: “Demonstration of convection in heated water”).
2. Do liquids also rise up when heated like air?
Ans: Yes, liquids rise when heated, similar to air. The heated water at the bottom becomes less dense, rises, and carries the potassium permanganate upward, creating convection currents (page 9: “In liquids… heat is transferred by… convection”).
Explanation: This illustrates convection in liquids, where heated water rises and cooler water sinks, forming currents (page 6, Fig. 7.5b). Potassium permanganate visualizes this movement, confirming that liquids, like gases, transfer heat via convection (page 9).
Activity 7.4: Let us investigate
- Caution—This activity should be carried out on a clear, sunny day under the supervision of a teacher or an adult.
- Take two identical bowls as shown in Fig. 7.6.
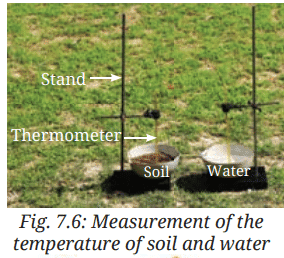
- Fill one bowl halfway with soil and the other bowl halfway with water.
- Fix a laboratory thermometer in each bowl as shown in Fig. 7.6. Make sure that the bulbs of the thermometer are immersed in soil and water, and do not touch the bottoms or the sides of the bowls.
- Place the set-up in sunlight.
- Measure the temperature of soil and water every 5 minutes and record the data in Table 7.4.
- Table 7.4: Temperature of soil and water when heated
- Study the rise in temperature of soil and water.
Ans: Measure the temperature of soil and water every 5 minutes and record the data in Table 7.4: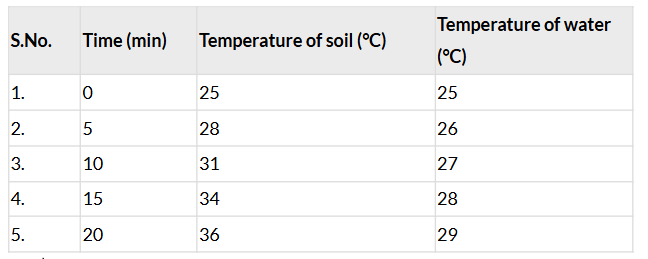
1. Did the temperature rise by the same amount for both the soil and the water at the same time?
Ans: No, the temperature did not rise by the same amount at the same time.
2. If not, which one got heated faster?
Ans: Soil heated faster than water (page 7: “Temperature of the soil rises more than that of water”).
3. How much was the rise in temperature of the soil and the water in 20 minutes?
- Soil: 36°C – 25°C = 11°C rise.
- Water: 29°C – 25°C = 4°C rise.
4. Does the soil also cool faster than water?
Ans: Yes, soil cools faster than water, as observed when the setup is brought indoors (page 7: “Soil cools faster than water”).
Explanation: This notes that soil heats and cools faster than water due to differences in specific heat capacity (page 7: “Soil heats up faster than water”). Simulated temperatures reflect this, with soil showing a greater temperature increase (11°C vs. 4°C). This explains sea and land breezes, as land’s rapid heating/cooling drives air movement (page 7, Figs. 7.7a, 7.7b).
Activity 7.5: Let us investigate
- Take three transparent, used plastic bottles of 1 L capacity.
- Cut them in the middle and make a small hole in the cap of each bottle.
- Keep them inverted and put some clay in one bottle, sand in the second, and gravel in the third, as shown in Fig. 7.10.
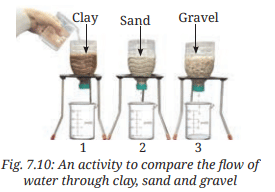
- Place three identical beakers below each bottle.
- Add 200 mL of water to each bottle.
- Table 7.5: Seepage of water
1. Predict the amount of water flowing out of each bottle:
Ans: Clay: Very slow (small particle size, low permeability). Sand: Slow (medium particle size, moderate permeability). Gravel: Fast (large particle size, high permeability).
2. Compare the amount of water that comes through each bottle and record in Table 7.5: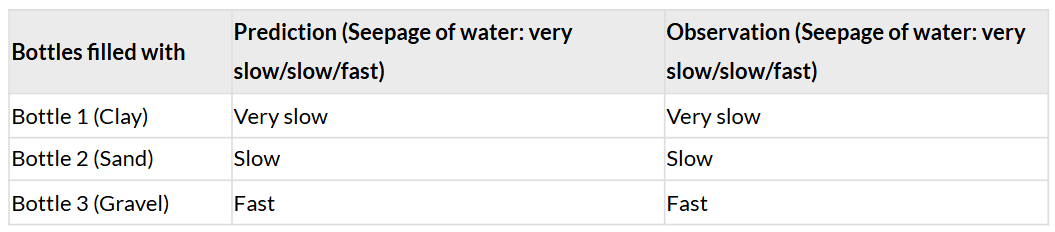
3. Explanation of observations:
Ans: Gravel allows the most water to flow due to large, open spaces between particles, followed by sand with smaller spaces. Clay, with tiny particles, retains water, allowing minimal seepage (page 11, Fig. 7.11: “Water readily moves… where spaces are wide”).
Explanation: This explains that water seeps through materials based on particle size and pore spaces (page 11). Gravel’s large spaces allow fast seepage, sand’s medium spaces allow moderate flow, and clay’s compact structure restricts flow, aligning with groundwater movement (page 12).
Exploratory Project: Paper Strip on Metallic Rod
Activity: Tightly wrap a thin paper strip around a metallic rod. Try to burn the paper with a candle while rotating the rod continuously. Does the paper burn? Explain your observations.
- Does the paper burn?
The paper does not burn easily while the rod is rotated continuously. - Explain your observations:
The metallic rod, a good conductor of heat, quickly absorbs and conducts the heat from the candle flame away from the paper. Continuous rotation ensures no single spot on the paper reaches the ignition temperature, preventing burning. This demonstrates conduction, as the metal transfers heat away from the paper (page 3: “Metals… allow heat to pass through them easily”).
Explanation: Metals’ high thermal conductivity (page 3) allows rapid heat dissipation. Rotating the rod prevents localized heating, keeping the paper below its ignition point, illustrating conduction’s role in heat transfer.
Exploratory Project: Spiral Paper Above a Burning Candle
Activity: Take a sheet of paper. Draw a spiral on it, as shown in Fig. 7.17a. Cut the paper along the spiral. Suspend the paper as shown in the Fig. 7.17b above a burning candle. Observe what happens. Provide an explanation for your observation.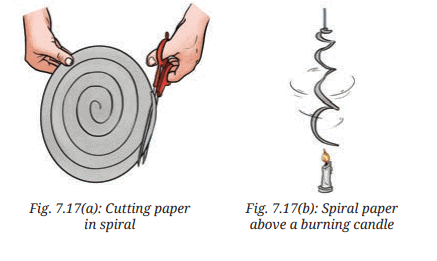
- Observe what happens:
The spiral paper rotates or rises above the burning candle. - Provide an explanation for your observation:
The candle flame heats the air above it, causing the air to expand, become less dense, and rise. This rising hot air creates convection currents that push against the spiral paper, causing it to rotate or lift (page 5: “As the air… warms up, it… rises up”). This demonstrates convection, where heat is transferred by the movement of heated particles (page 9).
Explanation: This describes convection as the movement of heated, less dense particles (page 9: “In convection, heat transfer… by the actual movement of particles”). The spiral’s motion is driven by rising hot air, similar to the rising cup in Activity 7.2 (page 5, Fig. 7.3b).
|
80 videos|224 docs|12 tests
|
FAQs on Class 7 Science Chapter 7 NCERT Based Activity - Heat Transfer in Nature
| 1. What is the purpose of the "Paper Strip on Metallic Rod" experiment? |  |
| 2. How does the "Spiral Paper Above a Burning Candle" experiment illustrate heat transfer? |  |
| 3. What types of heat transfer are explored in the NCERT activity on "Heat Transfer in Nature"? |  |
| 4. Why is it important to conduct experiments like those in Activities 7.1 to 7.5? |  |
| 5. What safety precautions should be taken during the heat transfer experiments? |  |
















