Class 7 Science Chapter 4 NCERT Based Activity - The World of Metals and Non-Metals
Activity 4.1: Let us explore
- Caution—Conduct this activity under the supervision of your teacher or an adult.
- Collect some waste pieces of copper and aluminium, an iron nail, a piece of coal, a pea-sized lump of sulfur (gandhak), and a block of wood.
- Recall the chapter 'Materials Around Us' in the Grade 6 Science textbook Curiosity and observe the appearances of the above items. Are they lustrous? Also, note whether they are hard or soft and record your observations in Table 4.1.
- Now, place each of these items one by one on any hard surface and beat them with a hammer (Fig. 4.2).
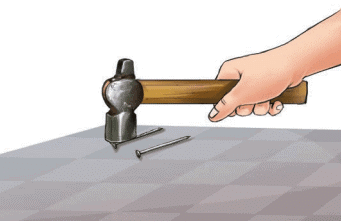 Fig. 4.2: Beating an iron nail with a hammer
Fig. 4.2: Beating an iron nail with a hammer - What do you think will happen? Do the objects become slightly flattened or do they break into pieces?
- Record your observations in Table 4.1.
1. Are they lustrous? Note whether they are hard or soft:
Ans: Based on the document (page 3), metals (copper, aluminium, iron) are lustrous and hard, while non-metals (coal, sulfur) and wood are non-lustrous and less hard.
2. What do you think will happen? Do the objects become slightly flattened or do they break into pieces?
Ans: Prediction: Metals (copper, aluminium, iron) will flatten due to malleability, while coal and sulfur will break into pieces (brittle), and wood will neither flatten nor break significantly (page 3–4).
Observation: As predicted, metals flatten, coal and sulfur break, and wood remains largely unchanged.
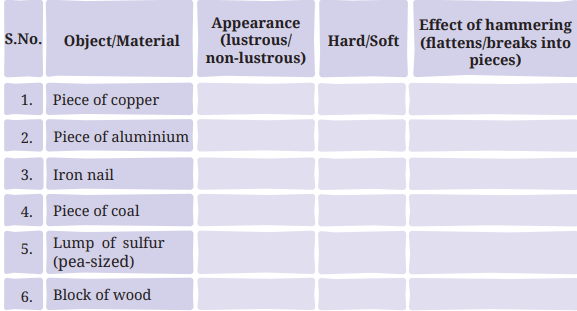
Table 4.1: Appearance, hardness, and effect of hammering on different objects or materials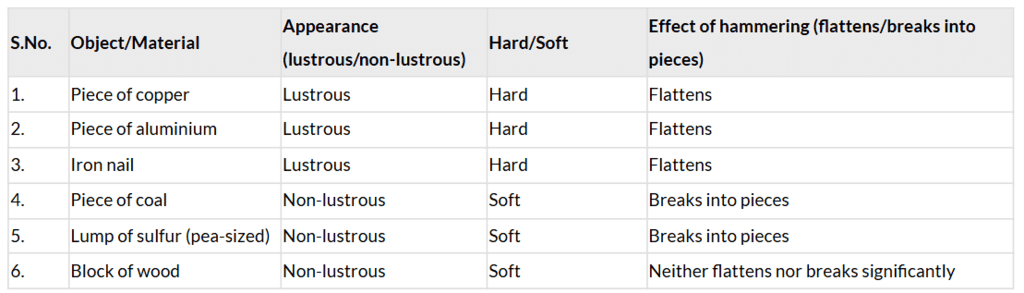
- Appearance: Metals (copper, aluminium, iron) are lustrous (page 3: “Lustre shown by metals is known as metallic lustre”). Coal, sulfur, and wood are non-lustrous (page 3).
- Hardness: Metals are hard, while coal, sulfur, and wood are softer (page 3: metals are “hard,” non-metals “not as hard”).
- Hammering: Metals are malleable and flatten (page 3: copper, aluminium, iron become flat). Coal and sulfur are brittle and break (page 4). Wood is neither malleable nor brittle, so it doesn’t flatten or break significantly (page 4).
Activity 4.2: Let us investigate
- Caution—Be careful while dropping the objects.
- Take a few objects, such as a metal spoon, a coin, a piece of coal, and a block of wood.
- Drop them one by one from a certain height.
- Do you notice any difference in the sound produced by these objects?
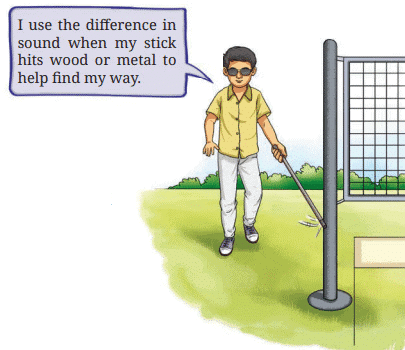 Ans: Yes, the metal spoon and coin produce a ringing sound, while the coal and wood produce dull sounds (page 6: “The metal spoon and the metal coin produce a ringing sound. Coal and wood, on the other hand, produce dull sounds”).
Ans: Yes, the metal spoon and coin produce a ringing sound, while the coal and wood produce dull sounds (page 6: “The metal spoon and the metal coin produce a ringing sound. Coal and wood, on the other hand, produce dull sounds”).
Explanation:
- This demonstrates sonority, a property of metals that causes them to produce a ringing sound when struck, unlike non-metals or wood (page 6: “This property of metals… is called sonority”).
Activity 4.3: Let us investigate
- Caution—This activity must be performed under the supervision of your teacher or an adult. Be careful while handling hot water.
- Place a glass tumbler on a table.
- Fill it with hot water.
- Take a metal spoon and a wooden spoon of almost the same size and thickness.
- Immerse both the spoons simultaneously into the hot water (Fig. 4.3) and leave them undisturbed for a few minutes.
- Now, carefully touch the upper end of each spoon.
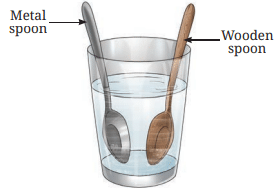 Fig. 4.3: Metal and wooden spoons immersed in hot water
Fig. 4.3: Metal and wooden spoons immersed in hot water
- Which of the spoons get hotter?
The metal spoon gets hotter than the wooden spoon. - What does this experiment tell us about heat transfer along the two spoons?
The experiment shows that heat transfers through the metal spoon, making it hotter, while the wooden spoon transfers heat poorly, remaining cooler. This indicates that metals are good conductors of heat, while wood is a poor conductor.
Explanation: Conduction is the transfer of heat through a material. Metals conduct heat well, so the metal spoon’s upper end becomes hot. Wood, a poor conductor, doesn’t transfer heat effectively (page 7).
Activity 4.4: Let us design and create
- Design an electric circuit, like the 'tester' circuit in the chapter 'Electricity: Circuits and their Components'. Repeat the same activity using the materials listed below and record your observations in Table 4.2.
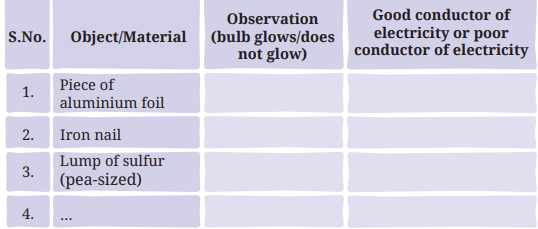
- You may collect a few objects, such as a piece of aluminium foil, an iron nail, a lump of sulfur (pea-sized), a copper wire, a piece of coal, a piece of dry wood, a stone, an eraser made of rubber and a piece of nylon rope.
Q: Predict which of these could make the bulb of the tester glow and which could not.
Ans: Prediction: Aluminium foil, iron nail, and copper wire (metals) will make the bulb glow, as they are conductors. Sulfur, coal, wood, stone, rubber eraser, and nylon rope (non-metals or non-conductive materials) will not make the bulb glow, as they are poor conductors (page 8).
Table 4.2: Conduction of electricity by different objects or materials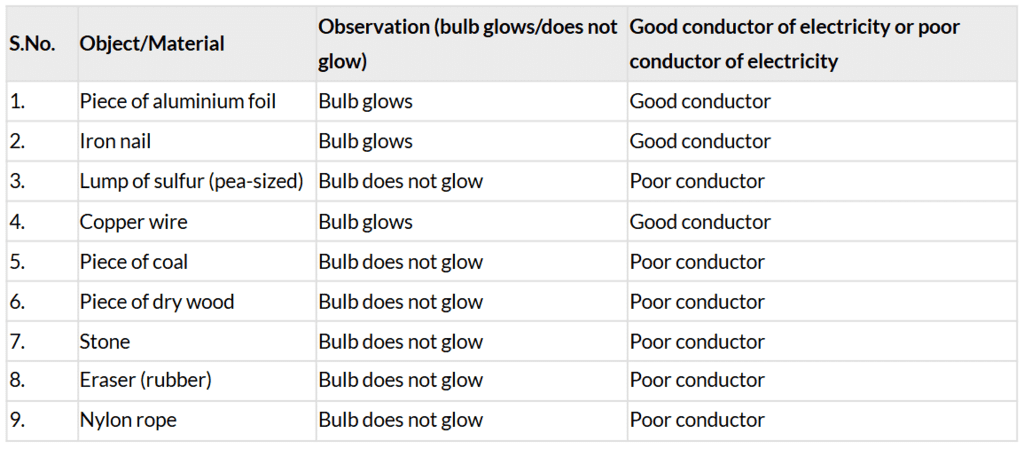
- The document states that aluminium, iron, and copper (metals) make the bulb glow, while sulfur, coal, wood, stone, eraser, and nylon rope do not (page 8: “Objects made of aluminium, iron, and copper make the bulb glow”).
- Metals are good conductors of electricity, allowing current to flow and light the bulb. Non-metals and other materials are poor conductors, preventing current flow (page 8).
Activity 4.5: Let us experiment
- Caution—Be careful while handling iron nails.
- Take a few shining iron nails. If you are using old iron nails, make sure to remove brown deposits from their surface by scrubbing them with the help of a small piece of sandpaper.
- Take three clean, dry glass bottles or test tubes with tight-fitting caps or stoppers. Label them A, B, and C.
- Take three iron nails and tie each iron nail with a thread.
- Place one iron nail and some silica gel in the glass bottle 'A', and tighten the cap or stopper (Fig. 4.4a). Silica gel makes the air dry. It is the substance that is used in small pouches in some medicine bottles, water bottles, shoe boxes, etc., to keep them dry.
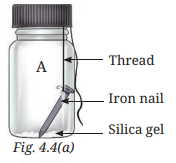
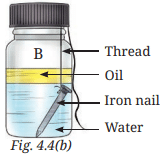
- Place one iron nail in the glass bottle 'B'. Pour freshly boiled and cooled water (to remove dissolved gases) into it until the iron nail is completely dipped in it. Now, pour some oil to form a layer over the surface of the water (Fig. 4.4b). The layer of oil on the surface of the water prevents the air from dissolving in the water. Cap the glass bottle tightly.
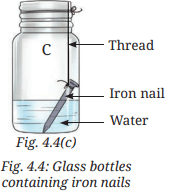
- Place one iron nail in the glass bottle 'C', and pour some water so that the iron nail is partially dipped. Keep this glass bottle unstoppered. This allows the iron nail to come into contact with both water and air, as shown in Fig. 4.4c.
- Place all the glass bottles undisturbed at room temperature and observe the changes for 8-10 days.
- Record your observations in Table 4.3.
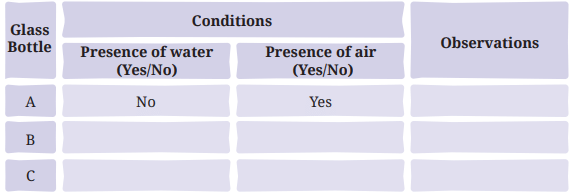
Ans: Table 4.3: Formation of brown deposit on iron nails
Q: What can you conclude from this experiment?
Ans: The presence of both water and air is essential for brown deposits (rust) to develop on iron nails. Rusting occurs only in bottle C, where the nail is exposed to moist air. In bottle A (dry air only) and bottle B (water only, no air), no rust forms (page 10: “The presence of both water and air is essential for these deposits to develop”).
Explanation: Rusting requires both water and air (moist air), as confirmed by the observation that only bottle C shows rust (page 10). Bottle A has dry air (silica gel absorbs moisture), and bottle B excludes air (oil layer prevents air contact), preventing rust.
Activity 4.6: Let us investigate (demonstration activity)
- The teacher may demonstrate this activity.
- Caution—It is advisable for students to wear protective eyeglasses and keep safe distance.
- Take a magnesium ribbon about 3-4 centimetres long. Clean it by rubbing with a piece of sandpaper.
- Hold it with a pair of tongs. Ignite the other end using a spirit lamp or a candle (Fig. 4.5).
- Let the magnesium ribbon burn.

- What do you observe?
- You must have observed that magnesium ribbon burns with a dazzling white flame and changes into a white powder. Collect it on a watch glass. This powder is magnesium oxide. It is formed due to the reaction between magnesium and oxygen present in the air.
- Add a few drops of warm water to this white powder, stir it well, and check its nature.
- Recall the chapter 'Exploring Substances: Acidic, Basic, and Neutral'. Find out whether the solution of magnesium oxide is acidic or basic or neutral in nature. You can use any acid-base indicator.
1. What do you observe (when burning magnesium ribbon)?
Ans: The magnesium ribbon burns with a dazzling white flame and changes into a white powder (magnesium oxide) (page 11).
2. Find out whether the solution of magnesium oxide is acidic or basic or neutral in nature:
Ans: The solution of magnesium oxide is basic in nature (page 11).
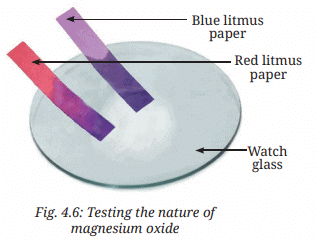
3. What effect does this solution have on blue and red litmus papers?
Ans: The solution turns red litmus paper blue and has no effect on blue litmus paper (page 11, Fig. 4.6).
Explanation: Magnesium reacts with oxygen to form magnesium oxide, which dissolves in water to form a basic solution (page 11: “Generally, oxides of metals are basic in nature”). A basic solution turns red litmus blue, as confirmed by Fig. 4.6.
Activity 4.7: Let us investigate (demonstration activity)
- The teacher may demonstrate this activity.
- Caution—This activity must be performed in a fume hood or well-ventilated area. Burning sulfur produces gases, which can be harmful if inhaled.
- Take a small amount of powdered sulfur in a deflagrating spoon (it is a long-handled metal spoon used in experiments to safely heat and burn substances Fig. 4.7a).

- If a deflagrating spoon is not available, you may take a metallic cap of any bottle, wrap a metallic wire around it and give it the shape as shown in Fig. 4.7b.

- Heat it on a flame, and as soon as the sulfur starts burning, introduce the deflagrating spoon into a gas jar or glass tumbler (Fig. 4.7c). Cover the gas jar or glass tumbler with a lid to ensure that the gas produced does not escape.
- Remove the lid after 3-4 minutes and take out the deflagrating spoon. Add a small quantity of water into the gas jar, quickly place the lid back and shake it so that the gas dissolves.
- Again, recall the chapter 'Exploring Substances: Acidic, Basic, and Neutral'. Using an acid-base indicator, check whether the solution obtained after the addition of water to the gas jar is acidic or basic or neutral.
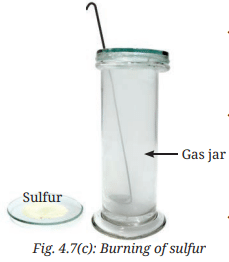
Q: What do you observe?
Ans: The solution obtained after dissolving the gas (sulfur dioxide) in water is acidic in nature (page 13, Fig. 4.7d).
Explanation: Burning sulfur produces sulfur dioxide gas, which dissolves in water to form sulfurous acid, an acidic solution (page 13: “On dissolving sulfur dioxide gas in water, sulfurous acid is formed”). This is confirmed by the acidic nature indicated by an acid-base indicator (e.g., turning blue litmus red).
Activity 4.8: Let us explore
- Take some sulfur powder in a glass tumbler.
- Add a small amount of water to it.
Q: What do you observe?
Ans: There is no reaction when sulfur is placed in water (page 13: “You may have noticed that there is no reaction when sulfur is placed in water”).
Explanation: Non-metals like sulfur generally do not react with water, unlike some metals (e.g., sodium), which aligns with the document’s statement about non-metals’ behavior (page 13).
|
85 videos|322 docs|12 tests
|
FAQs on Class 7 Science Chapter 4 NCERT Based Activity - The World of Metals and Non-Metals
| 1. What are metals and non-metals? |  |
| 2. How do metals and non-metals differ in their physical properties? |  |
| 3. What are some common uses of metals and non-metals? |  |
| 4. How do metals and non-metals react with acids? |  |
| 5. What is the significance of studying metals and non-metals in Class 7 science? |  |
















