| Table of contents |

|
| Short Answer Type Questions |

|
| Matching Type Questions |

|
| Assertion and Reason Type Questions |

|
| Long Answer Type Questions |

|
Short Answer Type Questions
Q.29. How do you account for the strong reducing power of lithium in aqueous solution?
Ans. Electrode potential is a measure of the tendency of an element to lose electrons in the aqueous solution.
It mainly depends upon the following three factors:
(1) Sublimation enthalpy
(2) Ionization enthalpy
(3) Enthalpy of hydration
The sublimation enthalpies of alkali metals are almost similar. Since Li has the smallest size, its enthalpy of hydration is the highest among alkali metals. Although ionization enthalpy of Li is the highest among alkali metals, it is more than compensated by the high enthalpy of hydration. Thus, Li has the most negative standard electrode potential (-3.04 V) and hence Li is the strongest reducing agent in aqueous solution mainly because of its high enthalpy of hydration.
(1) 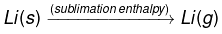
(2) 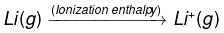
(3) Li+(g) + aq → Li+(aq) + enthalpy of hydration
Q.30. When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K.
Ans. The reactivity of alkali metals towards oxygen increases down the group as the atomic size increases. Thus, Li forms only lithium oxide (Li2O), sodium forms mainly sodium peroxide (Na2O2) along with a small amount of sodium oxide while potassium forms only potassium superoxide (KO2).
4Li + O2 → 2Li2O
2Na + O2 → Na2O2 + Na2O
K + O2 → KO2
This is because of the following two reasons:
(1) As the size of the metal cation increases, the positive field around it becomes weaker and weaker thereby permitting the initially formed oxide (O2-) ion to combine with another oxygen atom to from first peroxide ion (O2-) and then superoxide (O–2) ion.
(2) Since larger cations stabilize larger anions due to higher lattice energies, therefore, the stability increases from oxide → peroxide→superoxide as the size of the metal cation increases down the group and the size of the anion increases from oxide → peroxide → superoxide.
Q.31. Complete the following reactions
(1) O22- + H2O →
(2) O2- + H2O →
Ans. (1) Peroxide ions react with water and form H2O2
O22- + 2H2O → 2OH- + H2O2
(2) Superoxides react with water and form H2O2 and O2
2O-2 + 2H2O → 2OH- + H2O2 + O2
Q.32. Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Ans. (i) Both Li and Mg are harder and lighter than other elements in their groups.
(ii) Both form ionic nitrides Li3N and Mg3N2 by heating in an atmosphere of nitrogen.
Li resembles Mg due to similar atomic radii and ionic radii.
Q.33. Name an element from Group 2 which forms an amphoteric oxide and a water-soluble sulphate.
Ans. Due to small size and somewhat high ionization enthalpy of Be, Be(OH)2 is amphoteric in nature, i.e., it reacts with both acids and bases. Further, due to the small size, the hydration enthalpy of Be2+ ions is much higher than the lattice enthalpy of BeSO4. As a result, BeSO4 is highly soluble in water.
Q.34. Discuss the trend of the following:
(1) Thermal stability of carbonates of Group 2 elements.
(2) The solubility and the nature of oxides of Group 2 elements.
Ans. (1) All the alkaline earth metals form carbonates (MCO3). All these carbonates decompose on heating to give CO2 and metal oxide. The thermal stability; of these carbonates increases down the group, i.e., from Be to Ba,
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3
BeCO3 is unstable to the extent that it is stable only in atmosphere of CO2. It however shows reversible decomposition in closed container
BeCO3 ⇌ BeO + CO2
Hence, more is the stability of oxide formed, less will be the stability of carbonates. Stability of oxides decreases down the group. Since beryllium oxide is highly stable, it makes BeCO3 unstable.
(2) All the alkaline earth metals form oxides of formula MO. The oxides are very stable due to high lattice energy and are used as refractory material. Except BeO (predominantly covalent), all other oxides are ionic and their lattice energy decreases as the size of cation increases.
The oxides are basic and basic nature increases from BeO to BaO (due to increasing ionic nature).
BeO dissolves both in acid and alkalies to give salts and is amphoteric.
The oxides of the alkaline earth metals (except BeO and MgO) dissolve in water to form basic hydroxides and evolve a large amount of heat. BeO and MgO possess high lattice energy and thus are insoluble in water.
Q.35. Why are BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO4 and BaSO4 are insoluble?
Ans. The hydration enthalpies of BeSO4 and MgSO4 are quite high because of small size of Be2+ and Mg2+ ions. These hydration enthalpy values are higher than their corresponding lattice enthalpies and therefore, BeSO4 and MgSO4 are highly soluble in water. However, hydration enthalpies of CaSO4, SrSO4 and BaSO4 are not very high as compared to their respective lattice enthalpies and hence these are insoluble in water.
Q.36. All compounds of alkali metals are easily soluble in water, but lithium compounds are more soluble in organic solvents. Explain.
Ans. Because of the small size, high electronegativity and high ionization enthalpy, lithium compounds have considerable covalent character while compounds of other alkali metals are ionic in nature. As a result, compounds of lithium are more soluble in organic solvents while those of other alkali metals are more soluble in water.
Q.37. In the Solvay process, can we obtain sodium carbonate directly by treating the solution containing (NH4)2CO3 with sodium chloride? Explain.
Ans. No. In the Solvay process, ammonium hydrogen carbonate is prepared from ammonium carbonate, which then reacts with sodium chloride to form sodium hydrogen carbonate. Due to low solubility of NaHCO3, it gets precipitated and decomposes on heating to give Na2CO3.
We cannot obtain sodium carbonate directly by treating the solution containing (NH4)2CO3 with sodium chloride as both the products formed on reaction, i.e., Na2CO3 and NH4C1 are soluble and the equilibrium will not shift in forward direction.
(NH4)2CO3 + 2NaCl ⇌ Na2CO3 + 2NH4Cl
Q.38. Write Lewis structure of O2– ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
Ans. Lewis structure of O-2 is O - O- Oxygen atom with no charge has 6 electrons. Thus, its oxidation state is 0. Oxygen atom that carries negative charge has 7 electrons. Thus, its oxidation state is -1.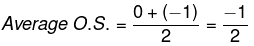
Q.39. Why do beryllium and magnesium not impart colour to the flame in the flame test?
Ans. All alkaline earth metals (except Be and Mg) impart a characteristic colour to the Bunsen flame. The different colours arise due to different energies. required for electronic excitation and de-excitation. Be and Mg atoms, due to their small size, bind their electrons more strongly (because of .higher effective nuclear charge). Hence, they require high excitation energy and are not excited by the energy of the flame with the result that no flame colour is shown by them.
Q.40. What is the structure of BeCl2 molecule in gaseous and solid-state?
Ans. Beryllium chloride has different structures in solid and vapour state. In solid-state, it exists in the form of polymeric chain structure in which each Be- atom is surrounded by four chlorine atoms, having two of the chlorine atoms covalently bonded while the other two by coordinate bonds. The resulting bridge structure contains infinite chains.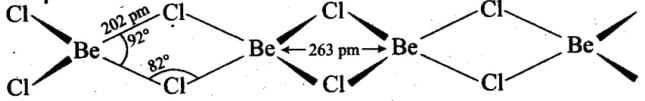 Structure of BeCl2 in Solid- State
Structure of BeCl2 in Solid- State
In Vapour state, above 1200 K, it exists as a monomer having linear structure and zero dipole moment. But below 1200 K, it exists as dimer even in the vapour state.
Matching Type Questions
In the following questions, more than one option of column I and II may be correlated.
Q.41. Match the elements given in Column I with the properties mentioned in Column II.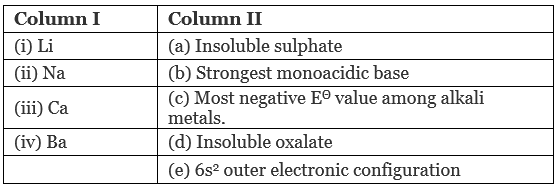
Ans. (i → c); (ii → b); (iii → d); (iv → a, e)
(i) Li – Most negative E° among alkali metals
[Due to very high hydration energy, the resulting E° is most negative].
(ii) Na – Strongest monoacidic base
[Alkalies are more acidic than alkaline earth metals. LiOH has covalent character]
(iii) Ca – Insoluble oxalate
[Calcium oxalate is insoluble in water.]
(iv) Ba – Insoluble sulphate
[Hydration energy decreases as the size of cation increases].
6s2 outer electronic configuration
56Ba = Is2, 2s2, 2p, 3s2, 3p6, 3d10, 4s2, 4p6, 4d10, 5s2, 5p6, 6s2
Q.42. Match the compounds given in Column I with their uses mentioned in Column II.
Ans. (i → c); (ii →d); (iii → b); (iv→a)
(i) CaCO3 – Manufacture of high-quality paper
(ii) Ca(OH)2 – Used in whitewashing
(iii) CaO – Manufacture of sodium carbonate from caustic soda
(iv) CaSO4 – Dentistry, ornamental work
Q.43. Match the elements given in Column I with the colour they impart to the flame given in Column II.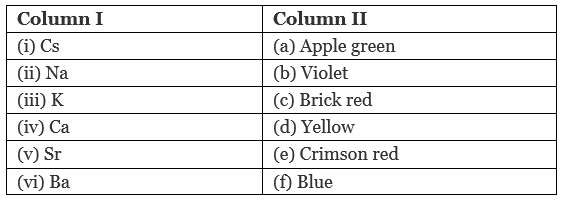
Ans. (i → f); (ii →d); (iii → b); (iv → c); (v →e); (vi → a)
Elements with the characteristic flame colour are as follows ‘
(i) Cs – Blue
(ii) Na-Yellow
(iii) K- Violet
(iv) Ca-Brick red
(v) Sr – Crimson red
(vi) Ba – Apple green
Flame colours are produced from the movement of the electrons in the metal ions present in the compounds. These movements of electrons (electronic excitation and de-excitation) requires energy.
Each atom has a particular energy gap between the ground and excited energy ‘ level. Therefore, each of these movements involves a specific amount of energy emitted as light energy, and each corresponds to a particular colour. As we know the energy gap between the ground and excited-state energy level increases, the wavelength of light absorbed decreases and the complementary colour is observed.
Assertion and Reason Type Questions
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Q.44. Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2.
Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) Both A and R are not correct
(d) A is not correct but R is correct.
Ans. (a)
Solution: Unlike other alkali metal carbonates, the carbonate of lithium decomposes on heating to form its oxide. Its oxide is stabilised by polarization.
Q.45. Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide.
Reason (R): Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) Both A and R are not correct.
(d) A is not correct but R is correct.
Ans. (a)
Solution: BeCO3 is kept in the atmosphere of CO2; otherwise it will decompose to give its oxide and carbon dioxide.
Long Answer Type Questions
Q.46. The s-block elements are characterised by their larger atomic sizes, lower ionisation enthalpies, invariable +1 oxidation state and solubilities of their oxo salts. In the light of these features describe the nature of their oxides, halides and oxo salts.
Ans.
(1) Nature of oxides – Alkali metals form M2O, M2O2 and MO2 types of oxides. The stability of the peroxide or superoxide increases as the size of metal cation increases. This is due to the stabilization of large anions by larger cations.
(2) Nature of halides – Alkali metal halides have general formula MX. All halides are soluble in water. LiF is very less soluble in water due to its high lattice energy. Their melting points and boiling points follow the trend – fluoride > chloride > bromide > iodide. This is because, with an increase in the size of the halide ion, lattice energy increases.
(3) Oxosalts – Oxosalts of alkali metals are generally soluble in water and thermally stable. As electropositive character increases down the group, stability of carbonates and bicarbonates increases.
Q.47. Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics:
(1) Tendency to form ionic/covalent compounds.
(2) Nature of oxides and their solubility in water.
(3) Formation of oxo salts.
(4) Solubility of oxo salts.
(5) Thermal stability of oxo salts.
Ans. 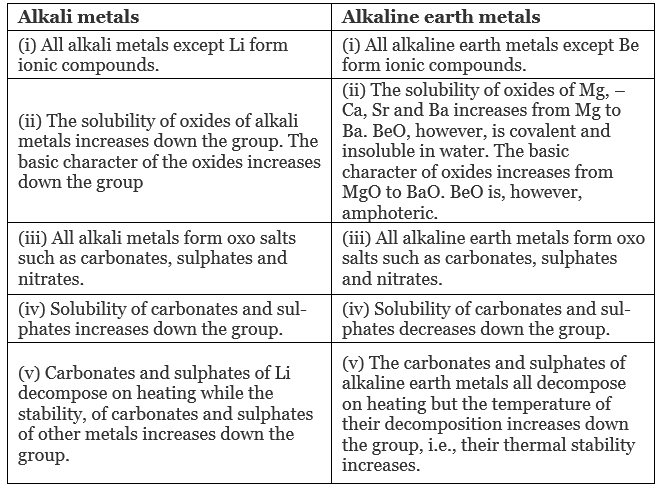
Q.48. When a metal of group 1 was dissolved in liquid ammonia, the following observations were obtained:
(1) Blue solution was obtained initially.
(2) On concentrating the solution, the blue colour changed to bronze colour.
How do you account for the blue colour of the solution? Give the name of the product formed on keeping the solution for some time.
Ans. (1) Alkali metals dissolve in liquid ammonia and give blue solution because of ammoniated electrons. These electrons absorb energy in the visible region of light and impart blue colour to the solution.
M+(x+y)NH3 → [M(NH3)x]+ + [e(NH3)y]- Ammoniated electrons.
(2) In concentrated solution, the blue colour changes to bronze colour due to the formation of clusters of metal ion. on standing, blue solution librates H2 gas with the formation of amide.
Q.49. The stability of peroxide and superoxide of alkali metals increases as we go down the group. Explain giving a reason.
Ans. The stability of peroxides and superoxides increases as the size of metal ion increases.
KO2 < RbO2 < C2O2
Li gives the only monoxide, Na gives peroxide and K, Rb and Cs give superoxide also. Peroxide ion and superoxide ion combine with large size of alkali metals—stability increases as the size of cation increases.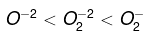
4Li + O2 →2Li2 (oxide)
2Na + O2 → Na2O2 (peroxide)
M+O2 → MO2 (superoxide)
(M= K,Rb,Cs)
Q.50. When water is added to compound (A) of calcium, solution of compound (B) is formed. When carbon dioxide is passed into the solution, it turns milky due to the formation of compound (C). If excess of carbon dioxide is passed into the solution milkiness disappears due to the formation of compound (D). Identify the compounds A, B, C and D. Explain why the milkiness disappears in the last step.
Ans. Solution B turns milky on passing CO2, it is lime water Ca(OH)2 and compound C which gives milky appearance is CaCO3. On passing excess of CO2 milkiness disappears due to the formation of compound D that is Ca(HCO3)2. Compound A reacts with water and gives B. It is CaO.
The reactions are:
CaO(A) + H2O → Ca(OH)2(B) lime water
Ca(OH)2(B)+ CO2 → CaCO3(C) calcium carbonate + H2O
Ca(OH)2(B) + CO2 + H20 → Ca(HCO3)2(D) calcium bicarbonate
Q.51. Lithium hydride can be used to prepare other useful hydrides. Beryllium hydride is one of them. Suggest a route for the preparation of beryllium hydride starting from lithium hydride. Write chemical equations involved in the process.
Ans. Beryllium hydride cannot be prepared directly by reaction with H2. It is prepared by reacting with LiAIH4.
Following reactions take place:
8LiH + Al2Cl6 → 2LiAlH4 - 6LiCl
2BeCl2 +LiAIH4 → 2BeH2 + LiCl + AlCl3
Q.52. An element of group 2 forms a covalent oxide which is amphoteric in nature and dissolves in water to give an amphoteric hydroxide. Identify the element and write chemical reactions of the hydroxide of the element with an alkali and an acid.
Ans. In group 2. He is the only element which gives covalent oxide BeO, which is amphoteric in nature. The rest elements of this group give ionic oxides which are basic in nature. BeO dissolves in water and gives sparingly soluble hydroxide which reacts with acid and base to give salt.
BeO + H2O → Be(OH)2
BE(OH)2 + 2OH- →[Be(OH)4]2-
Beryllate ion
BE(OH)2 + 2HCl + 2H2O → [Be(OH)4]Cl2
Q.53. Ions of an element of group 1 participate in the transmission of nerve signals and transport of sugars and amino acids into cells. This element imparts a yellow colour to the flame in a flame test and forms an oxide and a peroxide with oxygen. Identify the element and write a chemical reaction to show the formation of its peroxide. Why does the element impart colour to the flame?
Ans. Na+ ions participate in the transmission of nerve signals and give oxide and peroxide.
4Na + O2 → 2Na2O
2Na + O2 → Na2O2
2Na2O + O2 →2Na2O2
Ionisation enthalpy of sodium is low. When sodium metal or its salt is heated in Bunsen flame, the flame energy causes an excitation of the outermost electron which on reverting back to its initial position gives out the absorbed energy as visible light. That’s why sodium imparts yellow colour to the flame.
FAQs on NCERT Exemplar - s-block elements - 2 - JEE
| 1. What are s-block elements? |  |
| 2. What are the general characteristics of s-block elements? |  |
| 3. What is the electronic configuration of s-block elements? |  |
| 4. Why are s-block elements highly reactive? |  |
| 5. Give an example of the application of s-block elements in our daily lives. |  |














