NCERT Solutions for Class 7 Science - Acids, Bases and Salts
Q1. State differences between acids and bases.
Ans: Differences in Acid and Base:
Acid | Base |
Sour in taste | Bitter in taste |
Turns blue litmus paper red. | No effect on blue litmus paper. |
No effect on red litmus paper. | Turns red litmus paper to blue. |
No effect on turmeric paper. | Turns turmeric paper to red. |
Turns china rose solution to dark pink. | Turn china rose solution to green. |
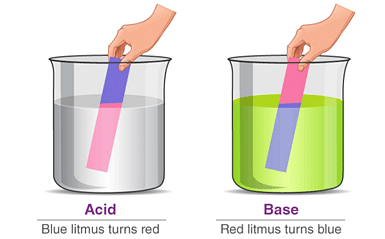
Q2. Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. What is its nature?
Ans: Since window cleaner turns red litmus paper to blue, it is basic in nature.
Q3. Name the source from which litmus solution is obtained. What is the use of this solution?
Ans: Litmus solution is obtained from lichens. Litmus solution is used to detect the acidic and basic characteristic of a substance.
Q4. Is the distilled water acidic/basic/neutral? How would you verify it?
Ans:
- Distilled water is neutral in character, i.e. it is neither acidic nor basic.
- Neutral nature of distilled water can be verified by the use of blue and red litmus paper.
- Distilled water does not change the colour of either blue or red litmus paper.
Q5. Describe the process of neutralization with the help of an example.
Ans: When the solution of acid is mixed with the solution of a base in the proper ratio, both of them neutralize the effect of each other, and a new substance; called salt; is formed; along with water. This is called neutralization or neutralization reaction.
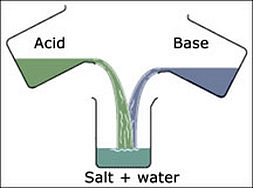
Example: When the solution of sodium hydroxide (a base) is mixed with the solution of hydrochloric acid (an acid) in the proper ratio, both neutralize each other and the reaction mixture so obtained is neutral in character. In this reaction, a new substance sodium chloride (common salt) is formed.
The reaction involved in this can be written as follows:
NaOH + HCl → NaCl + H2O + Heat
Q6. Mark ‘T’ if the statement is true and ‘F’ if it is false:
(i) Nitric acid turns red litmus blue. (T/F)
Ans: False
(ii) Sodium hydroxide turns blue litmus red. (T/F)
Ans: False
(iii) Sodium hydroxide and hydrochloric acid neutralize each other and form salt and water. (T/F)
Ans: True
(iv) Indicator is a substance which shows different colours in acidic and basic solutions. (T/F)
Ans: True
(v) Tooth decay is caused by the presence of a base. (T/F)
Ans: False
Q7. Dorji has a few bottles of soft drink in his restaurant. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink, another wants basic and third one wants neutral drink. How will Dorji decide which drink is to be served to whom?
Ans:
- Dorji would dip blue and red litmus paper in the sample of each of the bottles.
- Sample of solution of bottle which turns blue litmus paper red is acidic.
- Sample of solution of bottle which turns red litmus paper blue is basic.
- Sample of solution of bottle which does not change the colour of either blue or red litmus paper is neutral in nature.
- After detecting the acidic, basic and neutral nature of soft drink, Dorji would serve the drink to the customers according to their requirement.
Q8. Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
Ans: Antacid is a substance that works against acid. The antacid tablet is taken in the case of acidity to neutralize the excess acid produced in the stomach.
Antacid tablets neutralize the acid produced in the stomach and give relief from acidity.

(b) Calamine solution is applied on the skin when an ant bites.
Ans: Calamine solution, which is zinc carbonate, is a base. In the case of ant bites, ant injects an acid; called formic acid; in the skin which causes pain and irritation. By applying calamine solution, it neutralizes the effect of acid inject in the course of ant bite and gives relief from pain.
(c) Factory waste is neutralized before disposing it into the water bodies.
Ans: Most of the factory wastes contain acid. If the waste is flushed into the water as it is, then acid present in them would kill the aquatic organisms; along with creating pollution. Thus, it is necessary to neutralize the factory waste before disposing it into the water bodies.
Q9. Three liquids are given to you. One is hydrochloric acid, another is sodium hydroxide and third is a sugar solution. How will you identify them? You have only a turmeric indicator.
Ans: The following steps are taken to test the given liquids:
- Put a drop of provided liquid on the turmeric indicator. The solution that changes the colour of the indicator to red, is sodium hydroxide, which is basic in nature.
- Now, to make two mixtures, add a drop of sodium hydroxide on the other two liquids individually.
- The drop of each combination added to the turmeric indicator one after another.
- The mixture that changes the indicator to red colour includes a neutral solution of sugar.
- While the mixture contains hydrochloric acid that has been neutralized by the addition of sodium hydroxide, which does not show any colour change in the indicator.
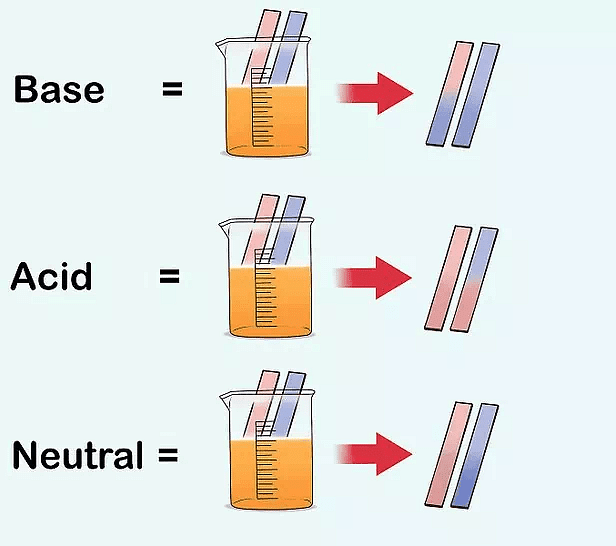
Q10. Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain.
Ans: If blue litmus paper is dipped in a solution and it would remain blue then the solution may be basic or neutral in character.
Blue litmus paper does not change its colour with basic solution and neutral solution.
Q11. Consider the following statements:
(a) Both acids and bases change the colour of all indicators.
(b) If an indicator gives a colour change with an acid, it does not give a change with a base.
(c) If an indicator changes colour with a base, it does not change colour with an acid.
(d) Change of colour in an acid and a base depends on the type of the indicator.
Which of these statements are correct?
(i) All four
(ii) a and d
(iii) b and c
(iv) only d
Ans: (iv) only d
|
111 videos|246 docs|28 tests
|
FAQs on NCERT Solutions for Class 7 Science - Acids, Bases and Salts
| 1. What are acids and bases? |  |
| 2. How do acids and bases react with each other? |  |
| 3. What is pH? |  |
| 4. What are some common uses of acids and bases? |  |
| 5. What are the properties of salts? |  |

|
Explore Courses for Class 7 exam
|

|


















