NCERT Solutions Class 12 Chemistry Chapter 2 - Electrochemistry
Page No.36 - Intext Questions
Q2.1: How would you determine the standard electrode potential of the system Mg2+ | Mg?
Ans: The standard electrode potential of Mg2+ | Mg can be measured with respect to the standard hydrogen electrode, represented by Pt(s), H2(g) (1 atm) | H (aq)(1 M).
A cell, consisting of Mg | MgSO4 (aq 1 M) as the anode and the standard hydrogen electrode as the cathode, is set up.
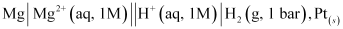
Then, the emf of the cell is measured and this measured emf is the standard electrode potential of the magnesium electrode.
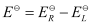
Here,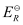 for the standard hydrogen electrode is zero.
for the standard hydrogen electrode is zero.
∴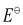
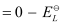
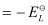
Q2.2: Can you store copper sulphate solutions in a zinc pot?
Ans: Zinc is more reactive than copper. Therefore, zinc can displace copper from its salt solution. If copper sulphate solution is stored in a zinc pot, then zinc will displace copper from the copper sulphate solution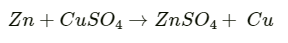
Hence, copper sulphate solution cannot be stored in a zinc pot.
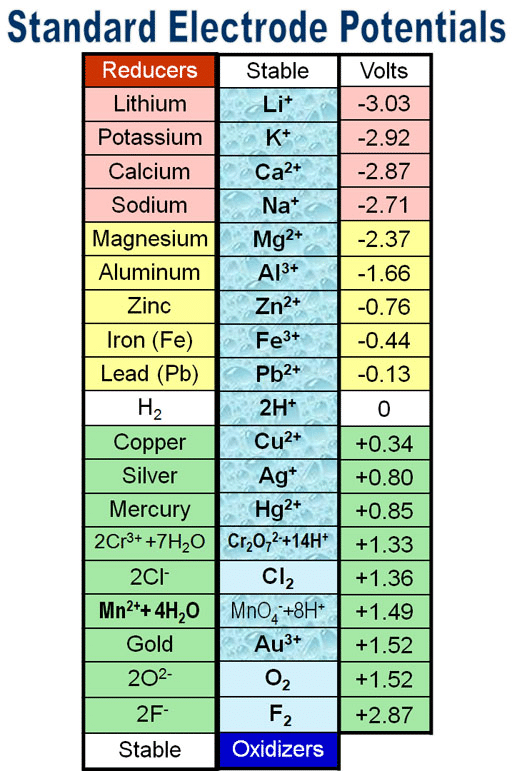
Q2.3: Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions
Ans: Substances that are stronger oxidising agents than ferrous ions can oxidise ferrous ions.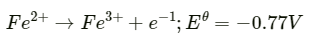
This implies that the substances having higher reduction potentials than
+0.77 V can oxidise ferrous ions to ferric ions. Three substances that can do so are F2, Cl2, and O2.
Page No.41 - Intext Questions
Q2.4: Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Ans: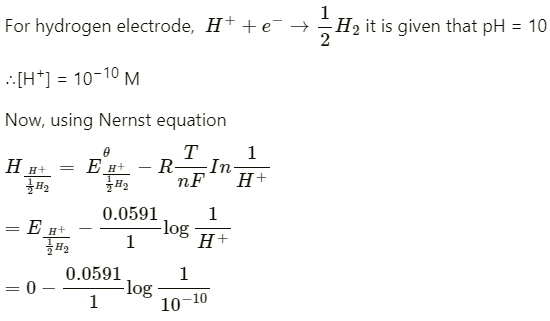
= −0.0591 log 1010
= −0.591 V
Q2.5: Calculate the emf of the cell in which the following reaction takes place: Ni(s)+2Ag+ (0.002 M) -> Ni2+ (0.160 M)+2Ag(s) Given that E(-)(cell) = 1.05 V
Ans: Applying Nernst equation we have: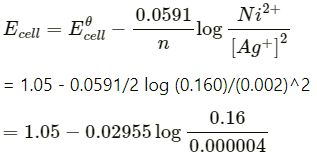
= 1.05 − 0.02955 log 4 × 104
= 1.05 − 0.02955 (log 10000 + log 4)
= 1.05 − 0.02955 (4 + 0.6021)
= 0.914 V
Q2.6: The cell in which the following reactions occurs: 2Fe3+ (aq) + 2I– (aq) —> 2Fe2+ (aq) +I2 (s) has E°cell=0.236 V at 298 K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Ans:
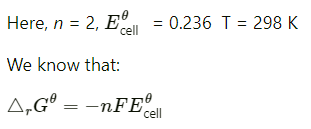
= −2 × 96487 × 0.236
= −45541.864 J mol−1
= −45.54 kJ mol−1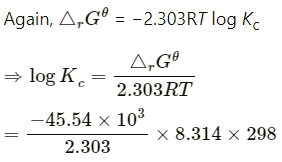
= 7.981
∴Kc = Antilog (7.981)
= 9.57 × 107
Page No.51 - Intext Questions
Q2.7: Why does the conductivity of a solution decrease with dilution?
Ans: Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution, the number of ions per unit volume decreases. Hence the conductivity decreases.
Q2.8: suggest a way to determine the 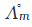 value of water.
value of water.
Ans: Λ∘m value of water.
Consider the following chemical equations,
(i) H2O(I) → H+(aq) + OH−(aq)
(ii) HCl(aq) → H+(aq) + Cl−(aq)
(iii) NaOH(aq) → Na+(aq) + OH−(aq)
(iv) NaCl(aq) → Na+(aq) + Cl−(aq)
Combining the four equations and using Kohlrausch's law, we get,
Λ∘m(H2O) = Λ∘m(HCl) + Λ∘m(NaOH) − Λ∘m(NaCl)
Hence, if we know the values of Λ∘m for HCl, NaOH and NaCl, we can calculate the value of Λ∘m for water.
Q2.9: The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree of dissociation and dissociation constant Given λ°(H+)=349.6 S cm2 mol-1 andλ°(HCOO-) = 54.6 S cm2 mol-1 .
Ans:
= 0.114 (approximately)
Thus, dissociation constant: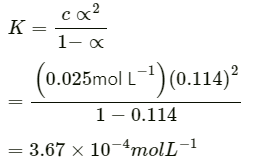
Q2.10: If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Ans: I = 0.5 A
t = 2 hours = 2 × 60 × 60 s = 7200 s
Thus, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that 96487C = 6.023 x 1023 number of electrons
Then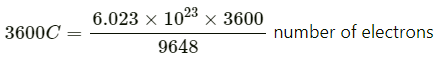
= 2.25 x 1022 number of electrons
Hence, 2.25 x 1022 number of electrons will flow through the wire.
Q2.11: Suggest a list of metals that are extracted electrolytically.
Ans: Metals that are on the top of the reactivity series such as sodium, potassium, calcium, lithium, magnesium, aluminium are extracted electrolytically.
Q2.12: Consider the reaction: Cr2O72--+ 14H+ + 6e- -> 2Cr3+ + 7H2O What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O72- ?
Ans: The given reaction is as follows: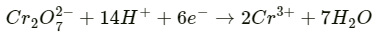
Therefore, to reduce 1 mole of 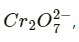 the required quantity of electricity will be:
the required quantity of electricity will be:
= 6 F
= 6 × 96487 C
= 578922 C
Page No.58 - Intext Questions
Q2.13: Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Ans: A lead storage battery consists of a lead anode, a grid of lead packed with lead oxide (PbO2) as the cathode, and a 38% solution of sulphuric acid (H2SO4) as an electrolyte.
When the battery is in use, the following cell reactions take place: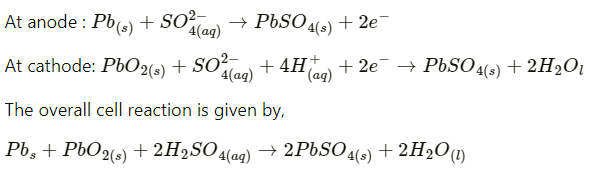
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging, 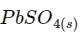 present at the anode and cathode is converted into
present at the anode and cathode is converted into 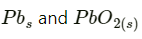 respectively.
respectively.
Q2.14: Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Ans: Methane and methanol can be used as fuels in fuel cells.
Q2.15: Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
Ans: In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode. The reaction at the anode is given by,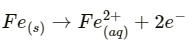
Electrons released at the anodic spot move through the metallic object and go to another spot of the object
There, in the presence of H+ ions, the electrons reduce oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is given by,
Also, ferrous ions are further oxidized by atmospheric oxygen to ferric ions. These ferric ions combine with moisture, present in the surroundings, to form hydrated ferric oxide 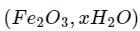 i.e., rust.
i.e., rust.
Hence, the rusting of iron is envisaged as the setting up of an electrochemical cell.
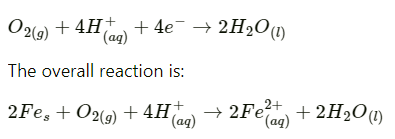
Exercises
Q2.1: Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn
Ans: The following is the order in which the given metals displace each other from the solution of their salts.
Mg, Al, Zn, Fe, Cu
Q2.2: Given the standard electrode potentials,
K+/K = −2.93V, Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = −2.37 V, Cr3+/Cr = − 0.74V
Arrange these metals in their increasing order of reducing power.
Ans: The lower the reduction potential, the higher is the reducing power. The given standard electrode potentials increase in the order of K+/K < Mg2+/Mg < Cr3+/Cr < Hg2+/Hg < Ag+/Ag.
Hence, the reducing power of the given metals increases in the following order: Ag < Hg < Cr < Mg < K
Q2.3: Depict the galvanic cell in which the reaction Zn(s) + 2Ag+ (aq) → Zn2+ (aq) 2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Ans: The galvanic cell in which the given reaction takes place is depicted as:
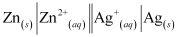
(i) Zn electrode (anode) is negatively charged.
(ii) Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
(iii) The reaction taking place at the anode is given by,
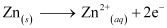
The reaction taking place at the cathode is given by,
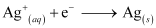
Q2.4: Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
(i) 2Cr(s) + 3Cd2+ (aq) → 2Cr3+ (aq) 3Cd
(ii) Fe2+ (aq) Ag+ (aq) → Fe3+ (aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
Ans: (i) 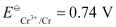
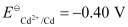
The galvanic cell of the given reaction is depicted as:
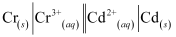
Now, the standard cell potential is
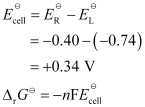
In the given equation,
n = 6
F = 96487 C mol−1
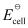 = 0.34 V
= 0.34 V
Then, 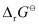 = −6 × 96487 C mol−1 × 0.34 V
= −6 × 96487 C mol−1 × 0.34 V
= −196833.48 CV mol−1
= −196833.48 J mol−1
= −196.83 kJ mol−1
Again,
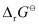 = −RT ln K
= −RT ln K
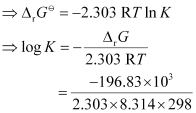
= 34.496
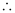 K = antilog (34.496)
K = antilog (34.496)
= 3.13 × 1034
(ii)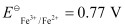
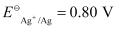
The galvanic cell of the given reaction is depicted as:
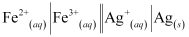
Now, the standard cell potential is
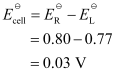
Here, n = 1.
Then, 
= −1 × 96487 C mol−1 × 0.03 V
= −2894.61 J mol−1
= −2.89 kJ mol−1
Again, 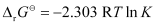
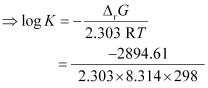
= 0.5073
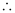 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)
Q2.5: Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s) | Mg2+ (0.001M) || Cu2+ (0.0001 M) | Cu(s)
(ii) Fe(s) | Fe2+ (0.001M) || H+ (1M)|H2(g)(1bar) | Pt(s)
(iii) Sn(s) | Sn2+ (0.050 M) || H+ (0.020 M) | H2(g) (1 bar) | Pt(s)
(iv) Pt(s) | Br2(l) | Br−(0.010 M) || H+ (0.030 M) | H2(g) (1 bar) | Pt(s).
Ans:
(i) For the given reaction, the Nernst equation can be given as:
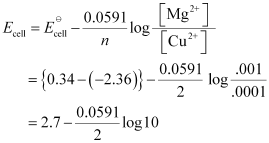
= 2.7 − 0.02955
= 2.67 V (approximately)
(ii) For the given reaction, the Nernst equation can be given as:
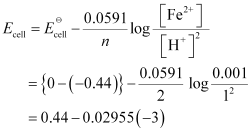
= 0.52865 V
= 0.53 V (approximately)
(iii) For the given reaction, the Nernst equation can be given as:
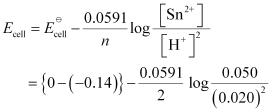
= 0.14 − 0.0295 × log125
= 0.14 − 0.062
= 0.078 V
= 0.08 V (approximately)
(iv) For the given reaction, the Nernst equation can be given as:
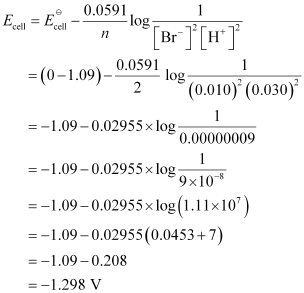
Q2.6: In the button cells widely used in watches and other devices the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH−(aq)
Determine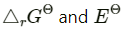 for the reaction.
for the reaction.
Ans:
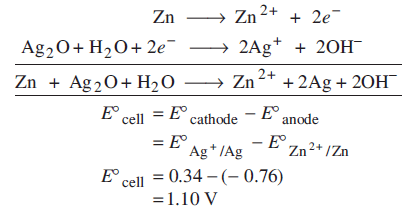
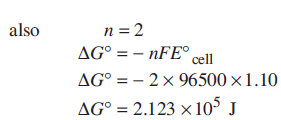
Q2.7: Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Ans: Conductivity : The reciprocal of resistivity is conductivity. Conductivity is the conductance of the electrolytic solution (due to the movement of ions and electrons) of one metre length and unit area of cross section. Mathematically, conductivity, K = 1/p
Conductivity varies with concentration. As the number of ions per cm3 of solution decreases, conductivity decreases.
Molar conductivity : The conductivity of one molar electrolytic solution is termed as molar conductivity. Mathematically,
Molar conductivity (Δm) = k/c,
where c = concemtration (in mol m-3)
K = conductivity (in S m-1)
It should be noted that for strong electrolytes there is only a small increase in Δm value with dilution. However for weak electrolytes, there is a large increase in Δm value with dilution.
Q2.8: The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 Scm−1. Calculate its molar conductivity.
Ans: Given,
κ = 0.0248 S cm−1
c = 0.20 M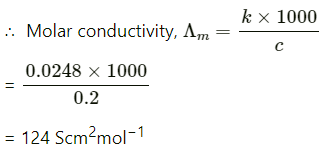
Q2.9: The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10−3 S cm−1.
Ans: Given,
Conductivity, κ = 0.146 × 10−3 S cm−1
Resistance, R = 1500 Ω
∴ Cell constant = κ × R
= 0.146 × 10−3 × 1500
= 0.219 cm−1
Q2.10: The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
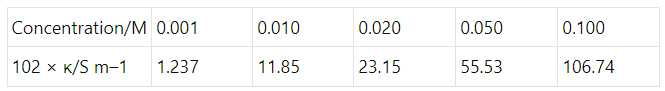
Calculate 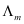 for all concentrations and draw a plot between
for all concentrations and draw a plot between 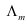 and c½. Find the value of
and c½. Find the value of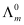 .
.
Ans: Given,
κ = 1.237 × 10−2 S m−1, c = 0.001 M
Then, κ = 1.237 × 10−4 S cm−1, c½ = 0.0316 M1/2
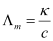
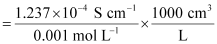
= 123.7 S cm2 mol−1
Given,
κ = 11.85 × 10−2 S m−1, c = 0.010M
Then, κ = 11.85 × 10−4 S cm−1, c½ = 0.1 M1/2
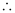
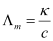
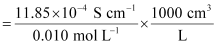
= 118.5 S cm2 mol−1
Given,
κ = 23.15 × 10−2 S m−1, c = 0.020 M
Then, κ = 23.15 × 10−4 S cm−1, c1/2 = 0.1414 M1/2
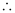
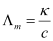
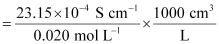
= 115.8 S cm2 mol−1
Given,
κ = 55.53 × 10−2 S m−1, c = 0.050 M
Then, κ = 55.53 × 10−4 S cm−1, c1/2 = 0.2236 M1/2
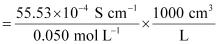
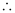
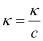
= 111.1 1 S cm2 mol−1
Given,
κ = 106.74 × 10−2 S m−1, c = 0.100 M
Then, κ = 106.74 × 10−4 S cm−1, c1/2 = 0.3162 M1/2
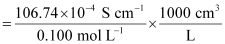
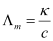
= 106.74 S cm2 mol−1
Now, we have the following data:
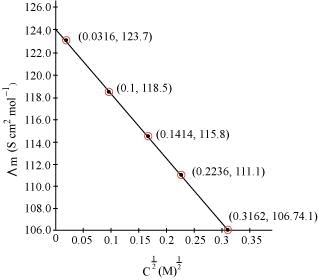
Since the line interrupts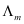 at 124.0 S cm2 mol−1,
at 124.0 S cm2 mol−1, 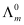 = 124.0 S cm2 mol−1.
= 124.0 S cm2 mol−1.
Q2.11: Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if 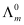 for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
Ans: Given, κ = 7.896 × 10−5 S m−1
c = 0.00241 mol L−1
Then, molar conductivity, 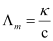
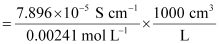
= 32.76S cm2 mol−1
Again, 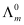 = 390.5 S cm2 mol−1
= 390.5 S cm2 mol−1
Now, 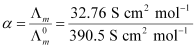
= 0.084
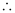 Dissociation constant,
Dissociation constant, 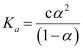
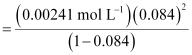
= 1.86 × 10−5 mol L−1
Q2.12: How much charge is required for the following reductions:
(i) 1 mol of Al3+ to Al.
(ii) 1 mol of Cu2+ to Cu.
(iii) 1 mol of Mno4 to MN2+
Ans: 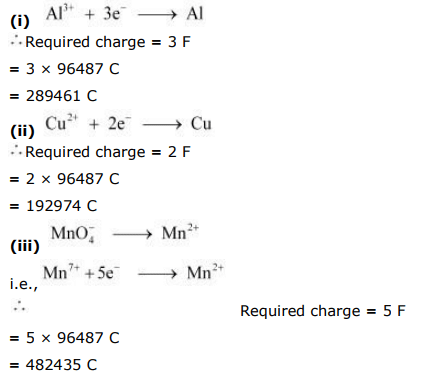
Q2.13: How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten CaCl2.
(ii) 40.0 g of Al from molten Al2O3.
Ans: (i) According to the question,
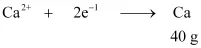
Electricity required to produce 40 g of calcium = 2 F
Therefore, the electricity required to produce 20 g of calcium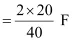
= 1 F
(ii) According to the question,
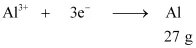
Electricity required to produce 27 g of Al = 3 F
Therefore, the electricity required to produce 40 g of Al 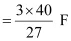
= 4.44 F
Q2.14: How much electricity is required in coulomb for the oxidation of
(i) 1 mol of H2O to O2.
(ii) 1 mol of FeO to Fe2O3.
Ans: (i) According to the question,
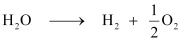
Now, we can write:
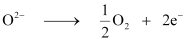
Electricity required for the oxidation of 1 mol of H2O to O2 = 2 F
= 2 × 96487 C
= 192974 C
(ii) According to the question,
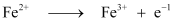
Electricity required for the oxidation of 1 mol of FeO to Fe2O3 = 1 F
= 96487 C
Q2.15: A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Ans: Given,
Current = 5A
Time = 20 × 60 = 1200 s
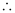 Charge = current × time
Charge = current × time
= 5 × 1200 = 6000 C
According to the reaction,
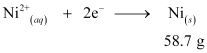
Nickel deposited by 2 × 96487 C = 58.71 g
Therefore, nickel deposited by 6000 C 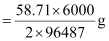 = 1.825 g
= 1.825 g
Hence, 1.825 g of nickel will be deposited at the cathode.
Q2.16: Three electrolytic cells A, B,C containing solutions of ZnSO4, AgNO3 and CuSO4, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Ans: According to the reaction:
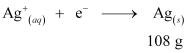
i.e., 108 g of Ag is deposited by 96487 C.
Therefore, 1.45 g of Ag is deposited by =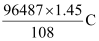 = 1295.43 C
= 1295.43 C
Given,
Current = 1.5 A
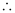 Time
Time 
= 863.6 s = 864 s
= 14.40 min
Again,

i.e., 2 × 96487 C of charge deposit = 63.5 g of Cu
Therefore, 1295.43 C of charge will deposit 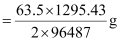
= 0.426 g of Cu
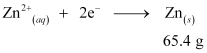
i.e., 2 × 96487 C of charge deposit = 65.4 g of Zn
Therefore, 1295.43 C of charge will deposit 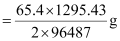
= 0.439 g of Zn
Q2.17: Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) Fe3+ (aq) and I−(aq)
(ii) Ag+ (aq) and Cu(s)
(iii) Fe3+ (aq) and Br− (aq)
(iv) Ag(s) and Fe3+ (aq)
(v) Br2 (aq) and Fe2+ (aq).
Ans:
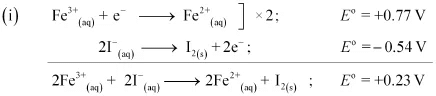
Since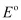 for the overall reaction is positive, the reaction between Fe3+ (aq) and I−(aq) is feasible.
for the overall reaction is positive, the reaction between Fe3+ (aq) and I−(aq) is feasible.
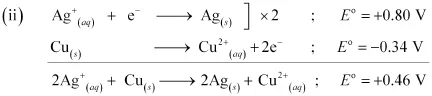
Since 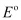 for the overall reaction is positive, the reaction between Ag+ (aq) and Cu(s) is feasible.
for the overall reaction is positive, the reaction between Ag+ (aq) and Cu(s) is feasible.
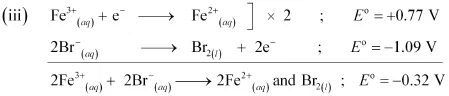
Since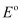 for the overall reaction is negative, the reaction between Fe3+ (aq) and Br−(aq) is not feasible.
for the overall reaction is negative, the reaction between Fe3+ (aq) and Br−(aq) is not feasible.
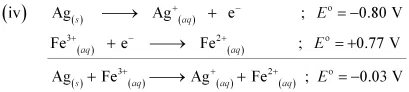
Since 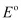 E for the overall reaction is negative, the reaction between Ag (s) and Fe3+ (aq) is not feasible.
E for the overall reaction is negative, the reaction between Ag (s) and Fe3+ (aq) is not feasible.
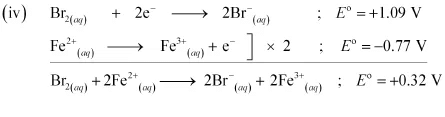
Since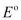 for the overall reaction is positive, the reaction between Br2(aq) and Fe2+ (aq) is feasible.
for the overall reaction is positive, the reaction between Br2(aq) and Fe2+ (aq) is feasible.
Q2.18: Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
(iii) A dilute solution of H2SO4 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Ans: (i) At cathode:
The following reduction reactions compete to take place at the cathode.
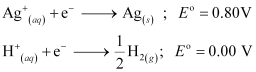
The reaction with a higher value of 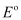 takes place at the cathode. Therefore, deposition of silver will take place at the cathode.
takes place at the cathode. Therefore, deposition of silver will take place at the cathode.
At anode:
The Ag anode is attacked by  ions. Therefore, the silver electrode at the anode dissolves in the solution to form Ag+.
ions. Therefore, the silver electrode at the anode dissolves in the solution to form Ag+.
(ii) At cathode:
The following reduction reactions compete to take place at the cathode.
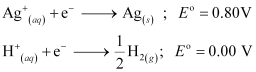
The reaction with a higher value of 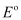 takes place at the cathode. Therefore, deposition of silver will take place at the cathode.
takes place at the cathode. Therefore, deposition of silver will take place at the cathode.
At anode:
Since Pt electrodes are inert, the anode is not attacked by  ions. Therefore, OH− or
ions. Therefore, OH− or  ions can be oxidized at the anode. But OH− ions having a lower discharge potential and get preference and decompose to liberate O2.
ions can be oxidized at the anode. But OH− ions having a lower discharge potential and get preference and decompose to liberate O2.

(iii) At the cathode, the following reduction reaction occurs to produce H2 gas.
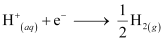
At the anode, the following processes are possible.
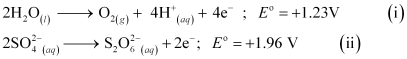
For dilute sulphuric acid, reaction (i) is preferred to produce O2 gas. But for concentrated sulphuric acid, reaction (ii) occurs.
(iv) At cathode:
The following reduction reactions compete to take place at the cathode.
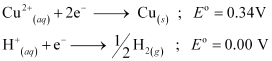
The reaction with a higher value of 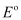 takes place at the cathode. Therefore, deposition of copper will take place at the cathode.
takes place at the cathode. Therefore, deposition of copper will take place at the cathode.
At anode:
The following oxidation reactions are possible at the anode.
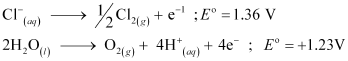
At the anode, the reaction with a lower value of 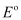 is preferred. But due to the over-potential of oxygen, Cl− gets oxidized at the anode to produce Cl2 gas.
is preferred. But due to the over-potential of oxygen, Cl− gets oxidized at the anode to produce Cl2 gas.
Old NCERT Solution
Q1: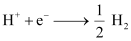 Calculate the potential of a hydrogen electrode in contact with a solution whose pH is 10.
Calculate the potential of a hydrogen electrode in contact with a solution whose pH is 10.
Ans: For hydrogen electrode, , it is given that pH = 10
∴[H ] = 10−10 M
Now, using Nernst equation:
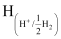 =
= 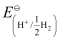
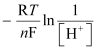
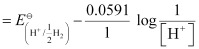
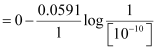
= −0.0591 log 1010
= −0.591 V
Q2: Calculate the emf of the cell in which the following reaction takes place:
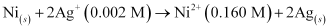
Given that 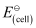 = 1.05 V
= 1.05 V
Ans: Applying Nernst equation we have:
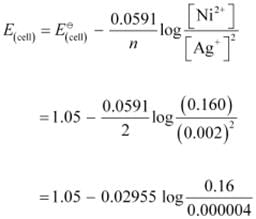
= 1.05 − 0.02955 log 4 × 104
= 1.05 − 0.02955 (log 10000 log 4)
= 1.05 − 0.02955 (4 0.6021)
= 0.914 V
Q3: The cell in which the following reactions occurs:
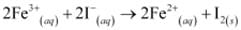 has
has 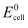 = 0.236 V at 298 K.
= 0.236 V at 298 K.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Ans: Here, n = 2, 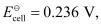 T = 298 K
T = 298 K
We know that:
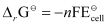
= −2 × 96487 × 0.236
= −45541.864 J mol−1
= −45.54 kJ mol−1
Again, 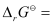 −2.303RT log Kc
−2.303RT log Kc
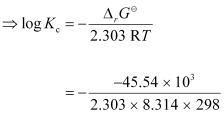
= 7.981
∴Kc = Antilog (7.981)
= 9.57 × 107
Q4: If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Ans: I = 0.5 A
t = 2 hours = 2 × 60 × 60 s = 7200 s
Thus, Q = It
= 0.5 A × 7200 s
= 3600 C
We know that 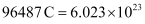 number of electrons.
number of electrons.
Then,
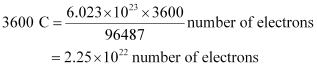
Hence, 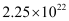 number of electrons will flow through the wire.
number of electrons will flow through the wire.
|
75 videos|278 docs|78 tests
|
FAQs on NCERT Solutions Class 12 Chemistry Chapter 2 - Electrochemistry
| 1. What is electrochemistry and why is it important? |  |
| 2. What are the basic components of an electrochemical cell? |  |
| 3. What is the difference between galvanic and electrolytic cells? |  |
| 4. How does the Nernst equation relate to electrochemistry? |  |
| 5. What factors affect the rate of an electrochemical reaction? |  |

















