Nitration, Sulphonation and Halogenation | Chemistry for JEE Main & Advanced PDF Download
Nitration of Benzene
Benzene reacts with concentrated nitric acid at 323-333K in the presence of concentrated sulphuric acid to form nitrobenzene. This reaction is known as nitration of benzene.
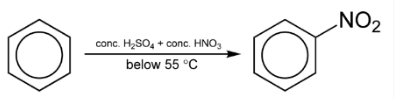
The mechanism for nitration of benzene:
Step 1: Nitric acid accepts a proton from sulphuric acid and then dissociates to form nitronium ion.
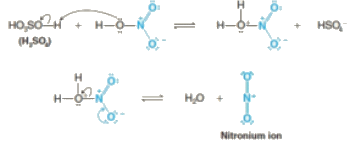
Step 2: The nitronium ion acts as an electrophile in the process which further reacts with benzene to form an arenium ion.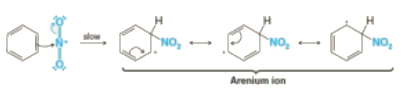
Step 3: The arenium ion then loses its proton to Lewis base forming nitrobenzene.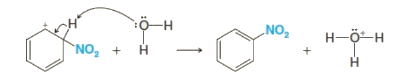
Sulphonation of Benzene:
Sulphonation of benzene is a process of heating benzene with fuming sulphuric acid (H2SO4 +SO3) to produce benzenesulphonic acid. The reaction is reversible in nature.
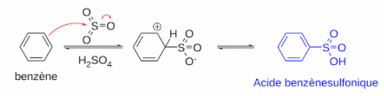
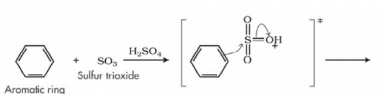
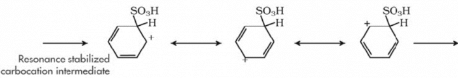
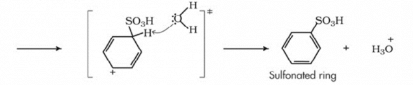
The mechanism for Sulphonation of benzene:
Due to higher electronegativity, oxygen present in sulphuric acid pulls an electron towards itself, generating an electrophile. This attacks the benzene ring, leading to the formation of benzenesulphonic acid.
Halogenation of Benzene
Benzene reacts with halogens in the presence of Lewis acid like FeCl3, FeBr3 to form aryl halides. This reaction is termed as halogenation of benzene.
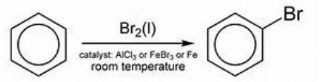
The mechanism for halogenation of benzene:
Step 1: Being a Lewis acid, FeBr3 helps in the generation of electrophile bromine ion by combining with the attacking reagent.
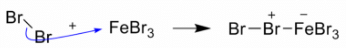
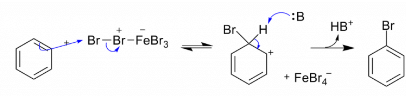
Step 2: The bromine ion acts as an electrophile in the process which further reacts with benzene to form arenium ion which finally converts to bromobenzene.
|
334 videos|660 docs|300 tests
|
FAQs on Nitration, Sulphonation and Halogenation - Chemistry for JEE Main & Advanced
| 1. What is nitration? |  |
| 2. What is sulphonation? |  |
| 3. What is halogenation? |  |
| 4. How are nitration, sulphonation, and halogenation useful in the production of pharmaceuticals? |  |
| 5. Are there any safety concerns associated with nitration, sulphonation, and halogenation processes? |  |

















