Redox Titrations | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Redox Titrations |

|
| Some Common Titration Methods |

|
| Redox Titration Experiment |

|
| Solved Examples of Redox Titrations |

|
Redox Titrations
Titration is a method used to figure out how strong or concentrated an unknown liquid is by slowly adding a known liquid strength to it.
Redox titrations involve the use of oxidizing and reducing agents to determine the concentration of a substance in a solution. The choice of reagents defines the type of redox titration. 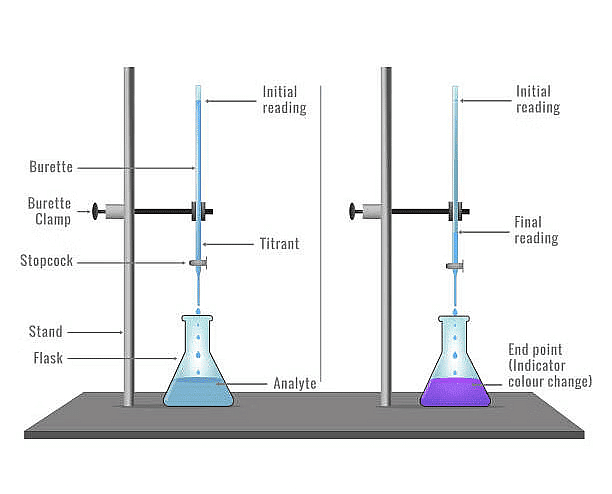 Redox Titration Setup
Redox Titration Setup
Some Common Titration Methods
1. Dichrometry:
- Potassium dichromate (K2Cr2O7) is the oxidizing agent.
- Redox indicator (e.g., barium diphenylamine sulphonate) is employed to indicate the titration endpoint.
- Example: Titration of iron(II) chloride with potassium dichromate.
6 FeCl2(aq) + K2Cr2O7(aq) + 14 HCl(aq) ⟶ 6 FeCl3(aq) + 2 CrCl3(aq) + 2 KCl(aq) + 7 H2O(l)
2. Permanganometry:
- Potassium permanganate (KMnO4) acts as both the oxidizing agent and indicator.
- No additional indicator is needed.
- Example: Titration of iron(III) with potassium permanganate using phosphoric acid to mask interfering color.
3. Iodimetric Titrations:
- Iodine (I2) is the oxidizing agent used to titrate reducing agents.
- Typically performed in neutral or slightly basic/acidic solutions.
- Example: The estimation of sodium bisulphite
 Estimation of Sodium Bisulphite
Estimation of Sodium Bisulphite
4. Iodometric Titrations:
- Iodide ion (I-) acts as a weak reducing agent.
- Different from iodimetric titrations which use iodine as the oxidizing agent.
 Example of Iodometric Titration
Example of Iodometric Titration
5. Bromatometry:
- Potassium bromate (KBrO3) serves as the oxidizing agent.
- Used in titrations to determine concentrations.
Redox Titration Experiment
Ever wondered how scientists test things in the lab? Let's take an example where they figure out how much hydrogen peroxide (H2O2) is in a store-bought bottle labeled 3%. They use a special solution called KMnO4 to do this. Here's how it works:
1. Weigh an empty flask with a precise balance.
2. Use a dropper to add 1.0 mL of the store-bought H2O2 into the flask and note the weight of the added liquid.
3. Pour 10mL of 3M H2SO4 and 50mL of water into the flask.
4. Load a tube with 50mL of a known solution (KMnO4) and write down how much is in the tube.
5. Place a white paper under the flask and slowly add the KMnO4 solution until the liquid in the flask changes color.
6. Note the amount of KMnO4 solution used by checking the difference between the initial and final levels in the tube.
7. Now, we can figure out the actual percentage of H2O2 in the store-bought bottle using some calculations.
In simpler terms, you're basically mixing stuff in a flask, adding a colored liquid until the mix changes color, and then doing some math to find out what's in the store-bought H2O2.
 |
Download the notes
Redox Titrations
|
Download as PDF |
Solved Examples of Redox Titrations
Q. In a titration of a 25.0 mL solution of Fe2+ with MnO4-, 16.7 mL of the 0.0152 M MnO4- was used. Find the concentration of Fe2+
Solution.
Step 1: Create a simple version of the equation that shows only the essential elements reacting.

Step 2: Figure out how many moles of MnO4- were involved in the reaction.

Step 3. Find the concentration of Fe2+ in the sample.

Q. Refer to the reactions below to observe how thiosulphate reacts differently with respect to iodine and bromine
2 S2O32– + I2 → S4O62– + 2I–
S2O32– + 2Br2 + 5H2O → 2SO42– + 2Br– + 10 H+
Select the statement that justifies the dual behavior of thiosulphate according to the above reactions.
- Iodine is a stronger oxidant than Bromine.
- Bromine is a stronger oxidant than iodine.
- Iodine undergoes reduction and bromine undergoes oxidation.
- Thiosulphate undergoes oxidation and reduction by bromine and iodine, respectively.
Solution. Option 2 is the correct answer. Bromine is a stronger oxidant than iodine. Bromine is a stronger oxidizing agent in comparison to iodine. Therefore, bromine oxidizes S of S2O32– to 2SO42– whereas I2 oxidizes S to S4O62–. Upon calculation, you will find that the oxidation number of S4O62– is less than 2SO42–.
|
353 videos|587 docs|309 tests
|





















