Revision Notes: Environmental Chemistry | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Atmospheric Pollution or Air Pollution |

|
| Stratospherical Pollution (Ozone Layer & Depletion) |

|
| Acid Rain |

|
| Greenhouse Effect & Global Warming |

|
| Water Pollution |

|
| Land Pollution |

|
Components of Environment:
- Atmosphere: This comprises a blanket of gaseous layer around earth.
- Hydrosphere: This comprises about 96% of earth’s surface & includes all sources of water like oceans rivers lakes, glaciers, ground water etc.
- Lithosphere: It refers to earth’s solid crust containing the outer mineral cover. It comprises soil, minerals, organic matter etc.
- Biosphere: It refers to the domain of living organism in covalent with atmosphere hydrosphere as well as lithosphere.
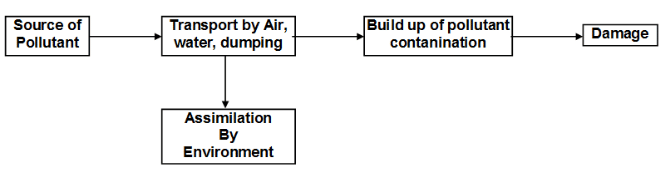
Environmental Pollution :
Process of contamination of the environment with harmful wastes arising mainly from human activities.
- Pollutant: Any substance or species produced either by a natural source or by human activity, which produces adverse effect on the environment.
- Contaminant: A substance which does not occurs in nature but is introduced by human activity into the atmosphere affecting its composition.
- Source: The site from which the pollution or contaminants originate.
- Sink: The material or medium which consumes or interacts with a long lived pollutant is called sink.
- Receptor : Anything that is affected by the pollutants.
- Threshold limit value (TLV) : This indicates the permissible limit of a pollutant in atmosphere to which a healthy worker is exposed during hours a day or 40 hours a week for life time without any adverse effects. TLV are determined by experimentation on animals, by use of medical knowledge, epidemiology surveys & environmental studies.
Atmospheric Pollution or Air Pollution
Tropospheric Pollution or Air Pollution:
- Definition: Atmospheric condition with concentrations causing harmful effects on humans and the environment.
- Substances:
- Gases: Oxides of sulfur, carbon monoxide (CO), nitrogen dioxide (NO2), hydrocarbons.
- Particulate Matter: Dust, smoke, fumes, etc.
- Radioactive Materials and others.
Primary Pollutants:
- Definition: Emitted directly from sources.
- Examples:
- Particulate Matter: Ash, smoke, dust, fumes, etc.
- Inorganic Gases: Sulfur dioxide, carbon monoxide, etc.
Secondary Pollutants:
- Definition: Formed in the atmosphere by chemical interactions among primary pollutants and normal atmospheric constituents.
- Examples:
- Sulphur trioxide, nitrogen dioxide, ozone, aldehyde, ketones.
- Various sulphate and nitrate salts.
Carbon Monoxide (CO)
Source:
- Incomplete combustion of carbonaceous matter, automobile engines, and defective furnaces.
- Incomplete combustion of fossil fuels, agricultural slash matter, and other carbon-containing sources.
Formation Reaction:
- 2C+O2→2CO
- Dissociation of carbon dioxide: 2CO2⇌2CO+O2
- Reaction of carbon dioxide with carbon-containing compounds at high temperature: C⇌2CO
Oxidation:
- Hydroxyl & perhydroxyl radicals, atomic oxygen, and ozone contribute to the oxidation of atmospheric CO into CO2.
Sink:
- Soil serves as a major sink for CO, where microorganisms help remove CO from the air.
Effect:
- Poisonous: Binds with hemoglobin in red blood cells, reducing oxygen transport efficiency.
- Health impact: Decreases oxygen transport to body organs and cells.
Carbon Dioxide (CO2)
Source:
- Released into the atmosphere by the combustion of fossil fuels (coal, oil) in factories and homes.
- Produced by the biological decay of plants.
Sink:
- Oceans act as a main sink for CO2.
Utilization:
- Utilized by green plants in photosynthesis.
Effect:
- Causes narcotic effect, stimulates respiratory centers, and can lead to asphyxiation.
- Contributes to climate change by raising general temperatures.
Oxides of Sulphur (SOx)
Source:
- Volcanic eruptions (natural activity) and combustion of sulfur-bearing fuels (coal, oil).
- Produced during roasting and smelting of sulfide ores (human activity).
Formation of Sulphuric Acid:
- Some SO2 undergoes photolytic and catalytic oxidation to form SO3, which converts to H2SO4 in the presence of moisture, causing acid rain.
Effect:
- Health impact: Causes cough, shortness of breath, larynx spasm, irritation to gas membranes, and reduced hearing ability.
- Harmful to respiratory systems, animals, plants, and materials (fabric, paper, leather).
Oxides of Nitrogen (NOx)
Source:
- NO2 produced by microbiological processes in soil and emitted into the atmosphere by natural activity.
Sink:
- Natural processes act as sinks for oxides of nitrogen.
Effects:
- Health impact: NO binds hemoglobin, reducing oxygen transport efficiency.
- Human health: Inhalation leads to pulmonary edema and hemorrhage.
- Plant damage: Causes leaf spotting and breakdown of plant tissues.
- Sunlight reaction: NO2 reacts with sunlight to produce highly active oxygen atoms.
Particulate matter:
Soot: produced by incomplete combustion of carbonaceous fossils fuels such as coal, fuel oil, natural gas, wood etc in insufficient supply of oxygen.
Metal particles: These are released by various metal finishing operation. The micro particles of toxic metal & SO2 gas present in the polluted atmosphere get absorbed on the particles rendering them highly toxic.
Metal oxides : They are generated by combustion of fuels containing metallic compounds.
Lead salts: Their source is lead tetraethyl (Pb(C2H5)4) which is added to gasoline to improve its antiknock property. In order to avoid deposition of PbO suitable amounts of C2H4Cl2 & C2H4Br2 are added to gasoline along with Pb(C2H5)4.
Fly ash: It originates from the combustion of high ash fossil. It contains partially burnt particles of the fuels.
Asbestos dust: It originates from industrial units manufacturing asbestos sheets, gaskets ropes etc. Asbestos flowing & asbestos insulations also contribute towards asbestos dust in the atmosphere.
Solid Hydrocarbons: These are emitted from petroleum refineries & comprise of paraffins, olefins & aromatics.
Dust Particulates: Originate from natural, domestic, industrial or agricultural sources. These are thrown into atmosphere by volcanic eruptions, blowing of dust by wind, mining operations etc.
Acid mist : Sulphuric acid mist is produced when SO3 present in the atmosphere comes in contact with moisture. Nitric acid mist is produced when oxides of nitrogen, viz, NO & NO2, undergo the series of reactions in the atmosphere.
Harmful effects of particulates
Effect on human beings: Affect the human respiratory system & cause several respiratory illnesses. The particles with small size are more harmful in this context. The particulates in fact, become the carriers of the toxic substances from the atmosphere to the human & cause big health hazards.
Effect on visibility: Particulates in the atmosphere cause scattering & absorption of sunlight & reduce the visibility.
Effect on Materials : The adverse effect of particulates on materials include corrosion of metals (when the atmosphere is humid), erosion & soiling of building, sculptures & painted surfaces & soiling of clothes & draperies.
Stratospherical Pollution (Ozone Layer & Depletion)
- Role of Ozone Layer: protecting earth from the UV radiation coming from the sun.
- Depletion of Ozone Layer : The equilibrium between formation & destruction of ozone has been upset by influx of several substances into the atmosphere which react with ozone to destroy it.
- Effect of Depletion of Ozone layer: The influx of UV radiation reaching the surface of earth would increase which would increase in risk to skin cancer due to exposure to UV radiation, UV radiations also tend to damage the immune system.
Acid Rain
Pollutants and Acid Formation:
- SO2 and Nitrogen Oxides: Atmospheric presence of sulfur dioxide (SO2) and nitrogen oxides.
- Interaction with Water Vapors: SO2 and nitrogen dioxide (NO2) interact with water vapors.
- Sunlight Presence: Reaction occurs in the presence of sunlight.
- Acid Formation: Results in the formation of sulfuric acid (H2SO4) and nitric acid (HNO3) units.
- Acidic Soots: Contribution to the formation of acidic particles in the atmosphere.
Greenhouse Effect & Global Warming
Greenhouse Effect:
- Formation of Thick Cover: CO2, CH4, O3, CFC’S create a thick layer around the Earth.
- Solar Energy Absorption: 75% of solar energy absorbed by Earth's surface.
- IR Radiation Absorption: Earth absorbs short-wavelength IR radiations from the sun.
- Temperature Rise: Earth emits longer-wavelength IR radiations, leading to rising temperatures.
- Greenhouse Gas Role: Greenhouse gases absorb Earth's infrared radiations, causing excessive atmospheric heating.
- Global Warming: Result of the enhanced greenhouse effect, trapping heat in Earth's atmosphere.
Advantages of Greenhouse Effect:
- Necessary Processes: Essential for evaporation, cloud formation, and rainfall.
- Promotes Growth: Warm atmosphere fosters rapid growth of plants and trees.
Harmful Effects of Greenhouse Effect:
- Melting Ice Caps: High temperatures may melt polar ice caps, raising sea levels and causing coastal destruction.
- Crop Reduction: Elevated temperatures can reduce crop yields.
- Reduced Work Efficiency: High temperatures may lower human work efficiency.
- Extreme Weather Events: Tropical rains and hurricanes may become more frequent and stronger, causing devastation.
- Ocean Impact: Changes in ocean temperature adversely affect marine life.
Water Pollution
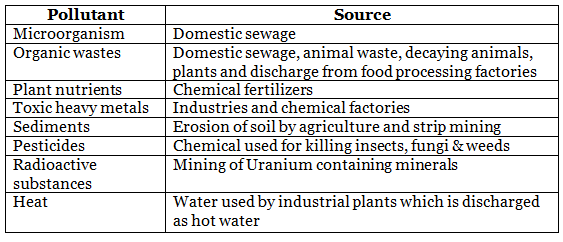
- Biochemical Oxygen Demand (BOD) measures total contamination that can be oxidized by microorganisms.
- It serves as a practical indicator of water quality.
- Clean water typically has a BOD value of less than 5 ppm.
- Highly polluted river water may exhibit a BOD value of 17 ppm or higher.
Land Pollution
- Pesticides and chemicals added to soil for better crop growth can have detrimental effects.
- Insecticides, a type of pesticide, are used to control insects, preventing diseases and protecting crops.
- Organo chlorines, such as DDT (dichlorodiphenyl trichloro ethane), are stable, toxic to insects but less harmful to humans, and not very soluble in water.
- The advantage of organo chlorines is their persistence in the environment.
- Fungicides are pesticides used to inhibit the growth of fungi.
- Fungi, lacking chlorophyll, cannot use solar energy for food preparation and may pose a threat to human interests.
- Fungi live as saprophytes on decaying organic matter or as parasites, affecting living organisms.
|
334 videos|651 docs|300 tests
|




















