Science Olympiad Model Test Paper - 1 | Olympiad Preparation for Class 10 PDF Download
Note: The questions provided in this document are similar to the questions that were asked in the actual Olympiad exam. So, we recommend you study these for your Olympiad preparation.
Logical Reasoning Section
Q1: If the first and last digits of each of the following numbers are swapped and then the numbers are sorted in ascending order, what will be the unit digit of the smallest number created?(a) 2
(b) 3
(c) 4
(d) 5
 View Answer
View Answer 
Ans: (c)
- To find the smallest number after swapping the first and last digits, we first need to perform the interchange on each number.
- After swapping, we arrange the new numbers in ascending order.
- The unit digit of the smallest number in this new arrangement will be our answer.
- Upon performing these steps, we find that the unit digit of the smallest number is 4.
Q2: P, Q, R, S, and T are arranged in a row facing North. P is positioned directly next to Q, while R is adjacent to S. S is not next to T, who occupies the leftmost position in the row. R is located in the second spot from the right. P is situated to the right of both Q and T. Who is positioned between Q and R?
(a) P
(b) S
(c) T
(d) Can't say
 View Answer
View Answer 
Ans: (a)
- To solve this, we first note that T is on the left end, meaning T is in position 1.
- Since R is in the second position from the right, it must be in position 4, leaving position 5 for S.
- With P next to Q and to the right of both Q and T, Q must be in position 2 and P in position 3.
- This arrangement shows that P is indeed sitting between Q and R.
Q3: Examine the information below carefully and respond to the question that follows: ‘A @ B’ signifies ‘A is the father of B’. ‘A + B’ indicates ‘A is the son of B’. ‘A $ B’ denotes ‘A is the daughter of B’. ‘A % B’ means ‘A is the mother of B’. ‘A & B’ implies ‘A is the husband of B’. Which of the following statements is accurate if the expression ‘P + Q % R $ S + T & W’ is certainly true?
(a) Q is the only daughter of T.
(b) P is the grandson of S.
(c) R is the granddaughter of W.
(d) T is the father-in-law of P.
 View Answer
View Answer 
Ans: (c)
- The expression ‘P + Q % R $ S + T & W’ can be broken down as follows:
- P is the son of Q.
- Q is the mother of R.
- R is the daughter of S.
- S is the mother of T.
- T is the husband of W.
- From this, we can conclude that R is indeed the granddaughter of W.
Q4: What number will come next in the given series?
102, 99, 93, 84, 72, ?
(a) 55
(b) 56
(c) 57
(d) 58
 View Answer
View Answer 
Ans: (c)
Each time the gap between the numbers is decreasing by 3.
99 – 102 = -3
93 – 99 = -6
84 – 93 = -9
72 – 84 = -12
To find the next term in the series, we need to do:
72 – 15 = 57
Q5: How many pairs of letters can be found in the word RESOURCES that have the same number of letters between them as they do in the English alphabet?
(a) 2
(b) 1
(c) 4
(d) 3
 View Answer
View Answer 
Ans: (a)
- To find the pairs of letters in the word RESOURCES, we need to check the distance between each letter and see if it matches their position in the English alphabet.
- For example, the letters R and T are 1 letter apart in the alphabet, and they are also 1 letter apart in the word.
- After checking all combinations, we find that there are 2 pairs that meet this criterion.
- Thus, the answer is 2.
Q6: Directions: Find the missing term in the following series.
10, 37, 101, 226, ?
(a) 432
(b) 442
(c) 369
(d) 378
 View Answer
View Answer 
Ans: (b)
The series is going up in cubes of continuous numbers, starting with the cube of 3.
10 + 33 = 37
37 + 43 = 101
101 + 53 = 226
226 + 63 = 442
Q7: If SCRIBBLE is coded as 09-11-17-23-06-06-18-24, then how will LIBBERS be coded in that language?
(a) 18-23-06-06-24-17-09
(b) 18-24-06-06-23-17-09
(c) 18-17-06-06-24-23-09
(d) 19-23-06-06-24-17-18
 View Answer
View Answer 
Ans: (a)
According to the question,
S → 09
C → 11
R → 17
I → 23
B → 06
B → 06
L → 18
E → 24
If we re-arrange SCRIBBLE, LIBBERS will be formed.
L→ 18
I → 23
B → 06
B → 06
E → 24
R → 17
S → 09
So, LIBBERS is coded 18-23-06-06-24-17-09.
Q8: In a certain code language, EMPTY is coded as FOSXD. How will STRING be coded in that language?
(a) TVUSMS
(b) TUVMSM
(c) TVUMSM
(d) TUVMMS
 View Answer
View Answer 
Ans: (c)
EMPTY is coded as FOSXD using the pattern:-
E + 1 = F
M + 2 = O
P + 3 = S
T + 4 = X
Y + 5 = D
In the same way, STRING will be coded as TVUMSM:-
S + 1 = T
T + 2 = V
R + 3 = U
I + 4 = M
N + 5 = S
G + 6 = M
Q9: Looking at a girl, Meera said, "She is the daughter of my grandfather's wife's only child." How is the girl related to Meera?
(a) Niece
(b) Mother
(c) Sister
(d) Cousin
 View Answer
View Answer 
Ans: (c)
Grandfather's wife → Meera's grandmother
Son of grandmother → Meera's father
Daughter of Meera's father → Meera's sister
Q10: A person is walking towards west. After walking for 30 m, he turns to his right and walks 20 m more. After that, he turns towards west and walks 40 m. Finally he turns to his left and again walks 20 m. How far is he now from the starting point?
(a) 40 m
(b) 50 m
(c) 60 m
(d) 70 m
 View Answer
View Answer 
Ans: (d)
S = Start point and E = End point.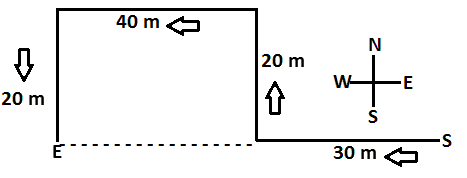
Science Section
Q11: A body weighs 500 gf in air and 300 gf when fully submerged in water. What are the apparent loss in weight, the upthrust on the body, and the volume of the body, respectively?(a) 200 gf, 200 gf, 200 cm³
(b) 200 gf, 200 gf, 100 cm³
(c) 100 gf, 100 gf, 100 cm³
(d) 200 gf, 400 gf, 200 cm³
 View Answer
View Answer 
Ans: (a)
- The apparent loss in weight of the body is calculated by subtracting the weight in water from the weight in air: 500 gf - 300 gf = 200 gf.
- This loss in weight is equal to the upthrust (or buoyant force) acting on the body, which is also 200 gf.
- To find the volume of the body, we use the relationship that 1 gf of weight corresponds to 1 cm³ of water displaced. Thus, the volume is 200 cm³.
- Therefore, the answers for the apparent loss in weight, upthrust, and volume are 200 gf, 200 gf, and 200 cm³, respectively.
Q12: A conducting wire shown in the figure carries current I. Segments AB and CD are of the same length. The direction of the magnetic field at point P is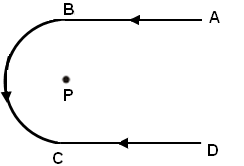 (a) into the plane of the paper
(a) into the plane of the paper
(b) out of the plane of the paper
(c) towards right
(d) towards left
 View Answer
View Answer 
Ans: (b)
- We can find the direction of the magnetic field by using the right thumb hand rule which states that if the thumb gives the direction of the current, then the direction of the magnetic field lines can be determined by the curling of the fingers.
- Here, the magnetic field at point P due to AB and CD segments will cancel each other and the magnetic field due to the semicircular section will be directed upwards (outward to the plane of paper).
Q13: Consider the following statements and select the option which correctly identifies true (T) and false (F) ones.
(i) Magnetic field lines do not intersect because they always travel parallel to each other in North to South direction.
(ii) When a current flows through a straight copper conductor, it gets permanently magnetized.
(iii) The strength of the magnetic field at the center of a circular coil is inversely proportional to the radius of the coil.
(a) T T F
(b) T F T
(c) F F T
(d) F T F
 View Answer
View Answer 
Ans: (c)
- Statement (i) is false because magnetic field lines do not travel parallel; they spread out from the North to South pole and cannot intersect.
- Statement (ii) is false as a straight copper conductor does not become permanently magnetized when current flows through it.
- Statement (iii) is true since the strength of the magnetic field at the center of a circular coil does decrease as the radius increases.
Q14: Which of the following statements about the given reaction is/are not correct?
Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s)
I. CuSO4 is getting reduced.
II. CuSO4 is acting as a reducing agent.
III. Fe is acting as a reducing agent.
IV. Fe is getting oxidised.
(a) III only
(b) I, II and IV
(c) II and IV
(d) I and III
 View Answer
View Answer 
Ans: (c)
- Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s)
- The given reaction is a displacement reaction as well as a redox reaction.
- The Cu+2 ion in CuSO4 is reduced to Cu. Hence, CuSO4 is acting as an oxidising agent.
- Fe is getting oxidised to Fe+2 in FeSO4. Hence, Fe is acting as a reducing agent.
- Therefore, statements II and IV are incorrect.
Q15: Safe current of a fuse wire depends upon
(a) its length
(b) its radius
(c) its colour
(d) its position in the circuit
 View Answer
View Answer 
Ans: (b)
Safe current through the fuse wire is independent of the length of the wire. It depends upon the radius of the fuse wire as I2 ∞r3.
Q16: Which of the following is a monoacidic base and a weak alkali?
(a) Ca(OH)2
(b) NaOH
(c) NH4OH
(d) Mg(OH)2
 View Answer
View Answer 
Ans: (c)
The bases which dissociate in aqueous solution to produce one mole of hydroxyl ion per mole of the base are called monoacidic bases.
For example, NaOH, KOH and NH4OH
NaOH ⇔ Na++ OH-
Ammonium hydroxide is a monoacidic base and can exist as either NH3 and H2O or NH4+ and OH- through a reversible reaction.
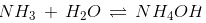
When ammonium hydroxide is allowed to reach equilibrium, far more NH3 and H2O exist compared to NH4+ and OH-, so the concentration of OH- is low; hence, ammonium hydroxide is considered to be a weak alkali.
Q17: A wire of the resistance 8Ω is cut in the ratio 3 : 1 and then two wires are connected across the 12 V battery. Then, the power developed across the larger length of the wire is
(a) 72 W
(b) 120 W
(c) 24 W
(d) 288 W
 View Answer
View Answer 
Ans: (c)
As the resistance of the wire is directly proportional to the length of the wire, the resistance of the larger length is 6Ω and the resistance of the smaller length is 2Ω.
P = V2 / R = 12 x 12 / 6
P = 24 W
Q18: Which of the following statements regarding metals and non-metals is/are correct?
I. Metals have 1 to 3 electrons in their valence shell and non-metals have 4-8 electrons in their valence shell.
II. Metallic oxides form acids on reaction with water and non-metallic oxides form bases.
III. Among the metals, the less electropositive metals displace the more electropositive metals from their salt solutions.
IV. Non-metals lose electrons from their valence shell to attain a stable configuration and form cations.
V. Metals like Au and Pt also form oxides readily.
(a) Only I
(b) Both I and II
(c) I, II and III
(d) I, III and V
 View Answer
View Answer 
Ans: (a)
The correct statements are as follows:
I. Metals have 1 to 3 electrons in their valence shell and non-metals have 4-8 electrons in their valence shell.
II. Metallic oxides form bases on reaction with water and non-metallic oxides form acids on reaction with water.
III. Among the metals, the more electropositive metals displace the less electropositive metals from their salt solutions.
IV. Non-metals accept electrons to attain a stable configuration and form anions.
V. Metals like Au and Pt are less reactive and do not form oxides.
Hence, option (a) is correct.
Q19: Which of the following molecules has the strongest carbon-to-carbon bond?
(a) C2H4
(b) C2H3
(c) C2H2
(d) C2H6
 View Answer
View Answer 
Ans: (c)
Order of bond strength of carbon-carbon bonds:
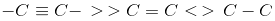
C2H2 is ethyne and has the structure 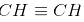 .
.
Hence, the carbon-carbon bond is the strongest in ethyne.
Q20: Which of the following is a non-commercial conventional source of energy?
(a) Coal
(b) Petroleum
(c) Firewood
(c) Electricity
 View Answer
View Answer 
Ans: (c)
Non-commercial energy sources include fuel wood, straw and dried dung. These are commonly used in rural India. According to an estimate, the total availability of fuel wood in India was only 50 million tonnes a year. It is less than 50% of the total requirement. In coming years, there would be a shortage of firewood.
Q21: Which of the following is NOT observed when calcium metal is dropped in water at room temperature?
(a) It reacts with water to liberate tiny bubbles of hydrogen gas.
(b) It sinks in water.
(c) It dissolves in water completely and instantaneously on account of the formation of calcium oxide.
(d) Water becomes warm.
 View Answer
View Answer 
Ans: (c)
Metals + H2O(l) Metal hydroxide + H2
Metals + H2O(g) Metal oxide + H2
Since calcium metal can react with water, it will yield a hydroxide.
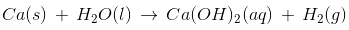
All the other observations are correct.
Q22: A girl standing in front of a large wall claps at a regular frequency of 12 Hz. She finds that the echoes coincide with her clapping. The speed of sound in air is 330 m/s. Which of the following statements is/are correct?
(i) The time taken between successive clappings is 0.2 s.
(ii) If she hears one more echo after she stops clapping, then the distance between the girl and the wall is 13.75 m.
(iii) The distance between the girl and the wall, after she stops clapping, if she hears four more echoes, is 55 m.
(a) (i) only
(b) (ii) and (iii) only
(c) (i) and (ii) only
(d) (i), (ii) and (iii)
 View Answer
View Answer 
Ans: (b)
- The time between claps: The frequency of 12 Hz means she claps every 1/12 seconds, which is 0.0833 s, not 0.2 s. So, statement (i) is incorrect.
- Distance calculation: The time for the echo to return after one clap is 0.2 s (0.1 s to the wall and 0.1 s back). The distance is calculated as speed × time = 330 m/s × 0.1 s = 33 m. Thus, statement (ii) is correct.
- Four echoes: If she hears four more echoes, it means 4 × 0.2 s = 0.8 s. The distance is 330 m/s × 0.4 s = 132 m, so statement (iii) is incorrect.
Q23: pH values of several solutions are provided:
Solution I: 2.2 – 2.4,
Solution II: 6.8,
Solution III: 7.4,
Solution IV: 10.
What could Solutions I, II, III, and IV be respectively?
(a) Vinegar, hydrochloric acid, window cleaner, and milk
(b) Curd, sodium hydroxide, milk of magnesia, and coffee
(c) Lemon juice, milk, human blood, and milk of magnesia
(d) None of these
 View Answer
View Answer 
Ans: (c)
- The pH scale measures how acidic or basic a solution is. A pH of 2.2 – 2.4 indicates a very acidic solution, which corresponds to lemon juice.
- A pH of 6.8 is slightly acidic and can be associated with milk.
- A pH of 7.4 is neutral and is typical for human blood.
- A pH of 10 indicates a basic solution, which is consistent with milk of magnesia.
Q24: Match column I with column II and select the correct option from the given codes.
Column I - Column II
(i) Components of ink - (P) Separating funnel
(ii) Petrol and water - (Q) Sublimation
(iii) Salt and ammonium chloride - (R) Fractional distillation
(iv) Separation of components of air - (S) Chromatography
(a) (i) - (S), (ii) - (P), (iii) - (Q), (iv) - (R)
(b) (i) - (P), (ii) - (R), (iii) - (S), (iv) - (Q)
(c) (i) - (Q), (ii) - (P), (iii) - (R), (iv) - (S)
(d) (i) - (R), (ii) - (Q), (iii) - (P), (iv) - (S)
 View Answer
View Answer 
Ans: (a)
- Components of inkchromatography, which is a method to separate mixtures.
- Petrol and waterseparating funnel.
- Salt and ammonium chloridesublimation, where one component turns into gas.
- Separation of components of airfractional distillation, which separates based on boiling points.
Q25: Observe the following reaction carefully. X + H⁺ → Y + H₂O + Sodium salt of acid (From acid) Now, select the correct statement.
(a) X is a mild corrosive acidic salt and can be used to neutralize a base.
(b) On heating X during cooking, H₂ gas is produced.
(c) X is used as an ingredient in antacids and is also used in soda-acid fire extinguishers.
(d) Y is carbon dioxide gas that makes bread and cakes soft and spongy.
 View Answer
View Answer 
Ans: (c)
- X is a substance commonly found in antacids, which help neutralize stomach acid.
- It is also used in soda-acid fire extinguishers, making it important for safety.
- Options (a) and (b) are incorrect because they misrepresent the properties of X.
- Option (d) incorrectly identifies Y; it is not carbon dioxide but rather a different product of the reaction.
Q26: Which of the following chemical equations represents a precipitation reaction?
(a) HCl + NaOH → NaCl + H2O
(b) 2KI + PbSO4 → PbI2 + K2SO4
(c) 2H2 + O2 → 2H2O
(d) None of these
 View Answer
View Answer 
Ans: (b)
In precipitation reactions, when a product formed by the displacement of anion or cation in the solution surpasses its point of saturation, then it precipitates as solid at the bottom of the solution.
For example, 2KI + PbSO4 → PbI2 + K2SO4
Here, lead and potassium are displaced from their compounds to form lead iodide that precipitates at the bottom of the container.
Q27: Which of the following pairs of elements indicates a mole ratio of 1:3?
(a) 12 g of carbon and 11 g of sodium
(b) 18 g of sulfur and 46 g of calcium
(c) 24 g of carbon and 12 g of magnesium
(d) 10 g of calcium and 18 g of magnesium
 View Answer
View Answer 
Ans: (d)
- To find the mole ratio, we need to calculate the number of moles for each element using the formula: moles = mass (g) / molar mass (g/mol).
- For calcium (Ca), the molar mass is about 40 g/mol, so 10 g of calcium is 0.25 moles.
- For magnesium (Mg), the molar mass is about 24 g/mol, so 18 g of magnesium is 0.75 moles.
- The ratio of moles of calcium to magnesium is 0.25:0.75, which simplifies to 1:3, confirming that option (d) is correct.
Q28: Which of the following is the incorrect IUPAC name for the specified compound?
(a) CH₃CH₂ – CO – CH₃ : Butanone
(b) CH₃CH₂ – CHO : Propanal
(c) CH₃CH₂ – COOCH₃ : Ethanoic acid
(d) CH₃– CH₂ – CH₂ – CH₂ – OH : Butanol
 View Answer
View Answer 
Ans: (c)
- The compound CH₃CH₂ – COOCH₃ is actually an ester, not an acid. The correct IUPAC name for this compound is ethyl acetate.
- Butanone (option a) is correctly named as it has a ketone functional group.
- Propanal (option b) is the correct name for the aldehyde with the structure CH₃CH₂ – CHO.
- Butanol (option d) is accurately named for the alcohol with the structure CH₃– CH₂ – CH₂ – CH₂ – OH.
Q29: Select the incorrect matches.
(i) Burning of natural gas – Exothermic reaction
(ii) Decomposition of vegetable matter into compost – Endothermic reaction
(iii) Reaction of zinc with copper sulfate – Decomposition reaction
(iv) Reaction of barium chloride with sodium sulfate – Single displacement reaction
(a) (ii), (iii) and (iv) only
(b) (ii) and (iii) only
(c) (i), (iii) and (iv) only
(d) (i), (ii) and (iv) only
 View Answer
View Answer 
Ans: (a)
- The question asks to identify the incorrect matches among the given reactions.
- Burning of natural gas is indeed an exothermic reaction, which releases heat.
- The decomposition of vegetable matter into compost is incorrectly labeled as endothermic; it is actually an exothermic process.
- The reaction of zinc with copper sulfate is a single displacement reaction, not a decomposition reaction.
- The reaction of barium chloride with sodium sulfate is a double displacement reaction, not a single displacement reaction.
Q30: An organic compound X has the molecular formula, C₂H₄O₂. X reacts with C₂H₅OH to produce Y. The reaction of X with sodium carbonate results in the formation of its corresponding salt, water, and a gas Z that produces a brisk effervescence. X, Y, and Z are respectively
(a) HCOOCH₃, CH₃COOCH₂CH₃, and H₂
(b) CH₃CH₂COOH, CH₃COOCH₃, and H₂
(c) CH₃COOH, CH₃COOCH₂CH₃, and CO₂
(d) CH₃COOH, CH₃COOCH₃, and O₂
 View Answer
View Answer 
Ans: (c)
- The compound X with the formula C₂H₄O₂ is acetic acid (CH₃COOH).
- When X reacts with C₂H₅OH (ethanol), it forms ethyl acetate (CH₃COOCH₂CH₃), which is Y.
- When X reacts with sodium carbonate, it produces a salt, water, and carbon dioxide (CO₂), which is the gas Z that causes effervescence.
- Thus, X, Y, and Z are acetic acid, ethyl acetate, and carbon dioxide, respectively.
Q31: Which of the following statements are incorrect?
I. Silver and copper are considered to be two of the best conductors of heat.
II. Alkali metals like lithium and sodium have high densities and high melting points.
III. Metals form acidic oxides while non-metals form basic oxides.
IV. Metals such as aluminum, iron, and zinc react with cold water to produce corresponding metal hydroxide and evolve hydrogen gas.
(a) I, II and III only
(b) II, III, and IV only
(c) I, III, and IV only
(d) II and IV only
 View Answer
View Answer 
Ans: (b)
- Statement I is correct; silver and copper are indeed excellent conductors of heat.
- Statement II is incorrect; alkali metals like lithium and sodium actually have low densities and low melting points.
- Statement III is incorrect; metals typically form basic oxides, while non-metals form acidic oxides.
- Statement IV is incorrect; metals like aluminum, iron, and zinc do not react with cold water.
In summary, the incorrect statements are II, III, and IV, making option (b) the correct choice.
Q32: The correct order of atomic radii for the given elements is:
(a) C > N > O
(b) C < N < O
(c) C > O > N
(d) C < O > N
 View Answer
View Answer 
Ans: (a)
C, N and O are elements of the second period.
The atomic radius decreases across the period because the effective nuclear charge increases across the period.
Hence, the correct order of the atomic radii is:
C > N > O
Q33: The ratio of atomic numbers of two elements C and D is 1:2. The number of electrons present in the valence shell (L-shell) of C is four less than the number of valence electrons present in the L-shell of D. Which of the following statements are correct regarding C and D?
I. The atomic numbers of C and D are 12 and 24 respectively.
II. The number of valence electrons in C and D are 3 and 7 respectively.
III. The difference between the number of neutrons of C and D is 3.
IV. The valency of both C and D is 2.
(a) I and IV only
(b) III and IV only
(c) II and III only
(d) I and II only
 View Answer
View Answer 
Ans: (b)
- The atomic numbers of C and D are in a ratio of 1:2, which means if C has an atomic number of 12, D must have 24. This makes statement I true.
- However, the number of valence electrons in C and D is not correctly stated in II, as C should have 4 and D should have 8, making II false.
- Statement III is true because if we assume C has 12 protons, it has 12 neutrons (for a stable isotope), and D has 24 protons, likely having 21 neutrons, leading to a difference of 3 neutrons.
- Statement IV is false because the valency of C and D is not 2; C has a valency of 4 and D has a valency of 2.
Q34: The electronic configuration of elements P, Q, R, and S are (2, 8, 2), (2, 8, 8, 2), (2, 6), and (2, 8, 6) respectively. Which of them can make an ion with two positive charges?
(a) P and R
(b) Q and S
(c) P and Q
(d) Q and R
 View Answer
View Answer 
Ans: (c)
- To form an ion with two positive charges, an element must lose two electrons.
- Element P has the configuration (2, 8, 2) and can lose 2 electrons from its outer shell.
- Element Q has the configuration (2, 8, 8, 2) and can also lose 2 electrons from its outer shell.
- Elements R and S cannot lose 2 electrons to form a +2 ion.
- Thus, the correct answer is P and Q, which can both form a +2 ion.
Q35: The energy absorbed by chlorophyll is responsible for carrying out which of the following functions?
(a) Formation of ATP
(b) Synthesis of NADPH
(c) Photolysis of water
(d) All of these
 View Answer
View Answer 
Ans: (d)
- Chlorophyll captures light energy during photosynthesis.
- This energy is crucial for multiple processes, including the formation of ATP, which provides energy for the plant.
- It also aids in the synthesis of NADPH, a molecule that carries electrons for chemical reactions.
- Additionally, chlorophyll facilitates the photolysis of water, which splits water molecules to release oxygen.
- Therefore, the correct answer is all of these functions are carried out by the energy absorbed by chlorophyll.
Q36: A tall pea plant with green seeds (TTyy) is crossed with a dwarf pea plant with yellow seeds (ttYY). All the progenies produced in the first generation will be
(a) Tall and yellow seeded
(b) Dwarf and yellow seeded
(c) Tall and green seeded
(d) Dwarf and green seeded
 View Answer
View Answer 
Ans: (a)
- The tall pea plant has the genotype TTyy, meaning it has two dominant alleles for height (T) and two recessive alleles for seed color (y).
- The dwarf pea plant has the genotype ttYY, which means it has two recessive alleles for height (t) and two dominant alleles for seed color (Y).
- When these plants are crossed, all offspring will inherit one allele from each parent, resulting in the genotype TtYy.
- This genotype expresses the tall phenotype due to the presence of the dominant T allele and the yellow seed color due to the dominant Y allele.
Q37: Which of these options represents denatured spirit?
(a) Pure ethanol
(b) Ethanol and methanol (5%)
(c) Ethanol and methanol (50%)
(d) Pure methanol
 View Answer
View Answer 
Ans: (b)
- Denatured alcohol is ethanol made unfit for human consumption by adding one or more chemicals (denaturants) to it. The additive may be any of the substances like methanol, copper sulphate, pyridine, etc.
- This practice is carried out to prevent the misuse of ethanol as an industrial raw material.
- Since the additives are added in a small quantity, option (2) is correct.
Q38: Freshly whitewashed walls develop a bright sheen on drying, which is due to the deposition of a substance that is similar in composition to
(a) austic potash
(b) limestone
(c) washing soda
(d) soap
 View Answer
View Answer 
Ans: (b)
Limewater is a saturated solution of calcium hydroxide which is used as a whitewash.
Atmospheric CO2 reacts with limewater to form calcium carbonate, which gives it a brilliant sheen.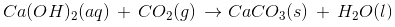
Q39: The following figure represents a chemical reaction, which can be categorised as a:
a. Decomposition reaction
b. Combination reaction
c. Displacement reaction
d. Oxidation reaction
(a) Only a
(b) Both b and c
(c) Both b and d
(d) Only d
 View Answer
View Answer 
Ans: (c)
S(s) + O2(g) → SO2(g)
This is a combination reaction as two reactants combine to give one product.
It is a redox reaction as sulphur is getting oxidised and oxygen is getting reduced.
Q40: Which of the following is an organic acid?
(a) Hydrochloric acid
(b) Suphuric acid
(c) Nitric acid
(d) Lactic acid
 View Answer
View Answer 
Ans: (d)
Organic acids are carboxylic acids, having an carboxyl group –COOH.
Lactic acid is an example of an organic acid.
Q41: Read the following statement and choose the option that accurately completes the blanks. The development of cartilage is
____(i)___ and it can be found in __(ii)__.
(a) Unidirectional, Middle part of the long bone
(b) Bidirectional, Blood vessels
(c) Unidirectional, Pinna of ears
(d) Bidirectional, Endoskeleton of vertebrates
 View Answer
View Answer 
Ans: (c)
- The growth of cartilage occurs in a unidirectional manner, meaning it grows in one direction.
- Cartilage is found in the pinna of the ears, which is the outer part of the ear.
- Understanding these points helps clarify the nature of cartilage growth and its locations in the body.
- Other options mention incorrect locations or growth patterns for cartilage.
Q42: The primary aim of water harvesting is not just to retain rainwater on the earth's surface but also to allow rainwater to seep underground to replenish groundwater. Which of the following statements are accurate regarding water stored underground?
(i) It helps recharge wells.
(ii) It does not encourage mosquito breeding.
(iii) It is used for the advantage of the local community.
(a) (i), (ii) and (iii)
(b) (i) and (iii) only
(c) (iii) only
(d) (i) and (ii) only
 View Answer
View Answer 
Ans: (a)
- Water stored underground plays a crucial role in recharging wells, which is essential for local water supply.
- It is beneficial as it does not promote mosquito breeding, helping to reduce health risks.
- This stored water is also utilized for the benefit of the local population, supporting agriculture and daily needs.
- Thus, all three statements (i), (ii), and (iii) are true regarding the advantages of underground water storage.
Q43: Select the incorrect pairing.
(a) Rhizobium - Converts atmospheric nitrogen gas into utilizable nitrogen compounds
(b) Azotobacter - Converts ammonia into nitrites
(c) Nitrobacter - Converts nitrites into nitrates
(d) Putrefying bacteria - Convert nitrogen-containing proteins of dead plants and animals into ammonia
 View Answer
View Answer 
Ans: (b)
- The question asks for the incorrect match among the options provided.
- Rhizobium is correctly known for converting atmospheric nitrogen into usable forms for plants.
- Nitrobacter accurately converts nitrites into nitrates, which is essential for plant nutrition.
- Putrefying bacteria play a role in breaking down nitrogen-containing proteins into ammonia.
- However, Azotobacter does not convert ammonia into nitrites; it actually fixes atmospheric nitrogen into ammonia, making option (b) the incorrect match.
Q44: Given below is a list of a few diseases.
(i) Typhoid
(ii) Dysentery
(iii) Cholera
(iv) Tuberculosis Which of these are transmitted by houseflies?
(a) (i) and (iii) only
(b) (ii), (iii) and (iv) only
(c) (i) and (iv) only
(d) (i), (ii), (iii) and (iv)
 View Answer
View Answer 
Ans: (d)
- Houseflies are known to carry and spread various diseases.
- Among the listed diseases, Typhoid, Dysentery, Cholera, and Tuberculosis can all be transmitted by houseflies.
- Houseflies can contaminate food and water, leading to the spread of these diseases.
- Therefore, the correct answer includes all the diseases listed: (i), (ii), (iii), and (iv).
Q45: What is the chemical name for Vitamin C?
(a) Folic Acid
(b) Biotin
(c) Ascorbic Acid
(d) Tocopherols
 View Answer
View Answer 
Ans: (c)
Vitamin C is also known as ascorbic acid or L-ascorbic acid.
It is a vitamin found in fresh fruits and vegetables, particularly citrus fruits and also taken as a dietary supplement.
Achievers Section
Q46: What are the end products formed when sulphuric acid reacts with sodium carbonate?(a) Carbon dioxide gas, Water, Sodium bicarbonate
(b) Carbon dioxide, Sodium sulphate
(c) Sodium sulphate, Water
(d) Sodium sulphate, Carbon dioxide gas, Water
 View Answer
View Answer 
Ans: (d)
Sulphuric acid gives sodium sulphate, carbon dioxide gas and water when it reacts with sodium carbonate.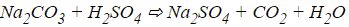
Q47: Read the given statements and select the option that correctly fills the blanks.
(i) ___ stimulates the transformation of the primary follicle of the ovary into the Graafian follicle.
(ii) Luteinizing hormone stimulates corpus luteum to secrete ___ hormone.
(iii) Ovulation is controlled by the increased level of ___ in blood.
(a) Follicle-stimulating, Progesterone, Luteinizing hormone
(b) Luteinizing, Progesterone, Estrogen
(c) Follicle-stimulating, Estrogen, Progesterone
(d) Luteinizing, Estrogen, Follicle-stimulating hormone
 View Answer
View Answer 
Ans: (a)
- Follicle-stimulating hormone (FSH) is responsible for the development of the primary follicle into the Graafian follicle.
- The luteinizing hormone (LH) triggers the corpus luteum to produce progesterone, which is crucial for maintaining pregnancy.
- Ovulation is primarily regulated by the rise in luteinizing hormone levels in the bloodstream.
- Thus, the correct sequence of hormones is FSH, progesterone, and LH, making option (a) the right choice.
Q48: Which of the following is not a limitation of Mendeleev's periodic table?
(a) Position of the noble gases
(b) Position of isotopes
(c) Position of hydrogen
(d) Anomalous pairs
 View Answer
View Answer 
Ans: (a)
The limitations of Mendeleev's periodic table are as follows:
1. Anomalous pairs, e.g. Co is placed before Ni even though Ni has a lower atomic mass.
2. Position of hydrogen is controversial as it resembles both alkali metals and halogens in many characteristics.
3. Position of isotopes is controversial as they were given the same slot, even though they have different atomic masses.
However, the noble gases could be accommodated in Mendeleev's table when they were discovered without disturbing the existing order.
Q49: Which of the following statements is NOT correct about the trends when going from left to right across the periods of the periodic table?
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
 View Answer
View Answer 
Ans: (c)
On moving from left to right across the periods of the periodic table, the atomic radius decreases and hence the ionisation energy increases.
Therefore, the tendency to lose the valence electrons, i.e. the electropositive character decreases.
Hence, option (c) is incorrect.
Q50: Read the given passage carefully and fill in the blanks by selecting an appropriate option. In the electrolytic refining process, the impure metal is made the (i)_____ and a thin strip of pure metal is made the (ii)_____. A solution of metal salt is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the (iii)_____ dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the (iv)_____. The insoluble impurities that settle down at the bottom of the anode are known as (v)_____.
(a) Anode, Cathode, Anode, Cathode, Anode mud
(b) Cathode, Anode, Cathode, Anode, Cathode mud
(c) Anode, Cathode, Cathode, Anode, Gangue particles
(d) Cathode, Anode, Anode, Cathode, Anode mud
 View Answer
View Answer 
Ans: (a)
- The impure metal acts as the anode in the electrolytic refining process.
- A thin strip of pure metal serves as the cathode.
- The electrolyte contains a solution of metal salt, which facilitates the process.
- As current flows, the pure metal from the anode dissolves into the electrolyte.
- Simultaneously, an equivalent amount of pure metal is deposited on the cathode, while the insoluble impurities settle at the bottom, referred to as anode mud.
|
70 videos|242 docs|187 tests
|
FAQs on Science Olympiad Model Test Paper - 1 - Olympiad Preparation for Class 10
| 1. What topics are covered in the Class 10 Science Olympiad Model Test Paper - 1? |  |
| 2. How can I prepare effectively for the Science Olympiad exam? |  |
| 3. What is the format of the Science Olympiad Model Test Paper? |  |
| 4. How important is the Logical Reasoning Section in the Science Olympiad? |  |
| 5. Where can I find additional resources for Science Olympiad preparation? |  |





















