Science Olympiad Model Test Paper - 2 | Science Olympiad Class 7 PDF Download
Note: The questions provided in this document are similar to the questions that were asked in the actual Olympiad exam. So, we recommend you study these for your Olympiad preparation.
Logical Reasoning Section
Q1: How many times does the digit 7 appear in the provided sequence, where it is directly followed by a symbol and directly preceded by the letter N?(a) 5
(b) 4
(c) 3
(d) 2
 View Answer
View AnswerAns: (d)
- To find the occurrences of 7, we need to look for instances where N is before the 7 and a symbol is after it.
- In the sequence, the valid occurrences are: N7%, N7#, and N7©.
- Counting these, we find there are 3 valid instances of 7 that meet the criteria.
- Thus, the correct answer is 2 as it is the only option that fits the criteria.
Q2: If Pintu is the brother of the son of Amar's son, how is Pintu related to Amar?
(a) Son
(b) Grandson
(c) Brother
(d) Father
 View Answer
View AnswerAns: (b)
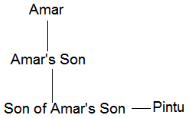 Son of Amar's son – Grandson
Son of Amar's son – Grandson
Brother of Amar's grandson – Amar's grandson
So, Pintu is Amar's grandson.
Q3: Raj is the son of Rani and the father of Sahil. If Meena is Sahil's mother, what is Meena's relationship to Rani?
(a) Daughter
(b) Mother
(c) Daughter-in-law
(d) Sister
 View Answer
View AnswerAns: (c)
- In this scenario, Raj is the son of Rani and the father of Sahil.
- Since Meena is identified as Sahil's mother, she is married to Raj.
- This makes Meena the daughter-in-law of Rani, as she is the wife of Rani's son.
- Thus, the correct answer is Daughter-in-law.
Q4: A person starts walking towards east. After walking 25 m, he turns to his right and walks 15 m. Again, he turns to his right and walks 25 m. How far is he now from the starting point?
(a) 15 m
(b) 25 m
(c) 40 m
(d) 65 m
 View Answer
View AnswerAns: (a)
Let S be the starting point and E be the end point.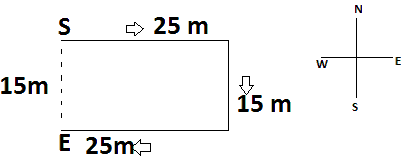
Q5: F is the mother of A. D is the daughter of E. If E is the husband of F, then which of the following relations is not definitely true?
(a) A is the daughter of E.
(b) D is the sister of A.
(c) F is the mother of D.
(d) E is the father of A.
 View Answer
View AnswerAns: (a)
- F is the mother of A and E is the husband of F. 'D is the daughter of E' means 'E is the father of D'.
- F is the mother of D. D is the sister of A.
- From the given information, we cannot get to know the gender of A.
So, A can be the daughter or son of E.
Q6: In a certain code, RUN is written as STO. How will RIVER be written in that code?
(a) SGWFS
(b) QIVGR
(c) QHWFS
(d) SHWDS
 View Answer
View AnswerAns: (d)
The code is as follows:
R + 1 = S
U - 1 = T
N + 1 = O
In the same way, RIVER will be written as follows:
R + 1 = S
I - 1 = H
V + 1 = W
E - 1 = D
R + 1 = S
Therefore, RIVER is coded as SHWDS.
Q7: In a specific coding system, if SCIENTIST is represented as VFJHQULVU, how will EDUCATION be encoded in that system?
(a) FEVDBUJPO
(b) HGVFDULRO
(c) HGUCDULRO
(d) FGVGEVLPO
 View Answer
View AnswerAns: (b)
- The coding system involves shifting each letter in the word by a certain number of positions in the alphabet.
- For example, in the word SCIENTIST, each letter is shifted to create the coded version VFJHQULVU.
- Applying the same shifting pattern to EDUCATION, we find that it translates to HGVFDULRO.
- This method of encoding is consistent, making option (b) the correct answer.
Q8: Find the water image of the following picture:
 (a)
(a) 
(b) 
(c) 
(d) 
 View Answer
View AnswerAns: (b)
The water image will be as follows:
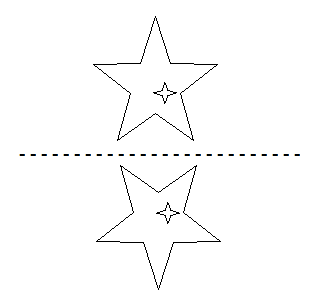
Q9: Find the mirror image of the following picture:
 (a)
(a) 
(b) 
(c) 
(d) 
 View Answer
View AnswerAns: (d)
The mirror image will be as follows:
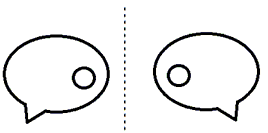
Q10: A bus is traveling in a West direction. It travels 7 km West, then makes a right turn and goes 5 km. After that, the bus makes another right turn and travels 19 km. What is the distance of the bus from its original starting point?
(a) 12 km
(b) 10 km
(c) 13 km
(d) 16 km
 View Answer
View AnswerAns: (c)
- The bus starts by moving 7 km West.
- After turning right, it moves 5 km North.
- Then, it takes another right turn and travels 19 km East.
- To find the distance from the starting point, we can visualize the path: it ends up 12 km East and 5 km North from the starting point.
- Using the Pythagorean theorem, the distance is calculated as √(12² + 5²) = √(144 + 25) = √169 = 13 km.
Science Section
Q11: Which of the following is not correct about thermometer?
(a) Thermometer is a device which is used to measure temperature.
(b) Thermometer is made of a long narrow glass tube, with a bulb at one end.
(c) The narrow tubes are filled with mercury.
(d) The narrow tube appears as a continuous golden line.
 View Answer
View AnswerAns: (d)
Thermometer is a device which is used to measure temperature. Thermometer is made of a long narrow glass tube. with a bulb at one end. The narrow tube appears as a continuous silver line because it is filled with mercury. Mercury is a metal which is in liquid state at room temperature and it readily expands or contracts at the slightest change in temperature. So, option (4) is the correct answer.
Q12: Which one of the following is an example of uniform motion?
(a) A train entering a railway platform.
(b) An aeroplane flying at a speed of 600 km/h.
(c) A bee going from one flower to another collecting nectar.
(d) A boy cycling in a busy street.
 View Answer
View AnswerAns: (b)
- Uniform motion means moving at a constant speed in a straight line.
- In this case, the aeroplane flying at a speed of 600 km/h is an example of uniform motion because it maintains a steady speed.
- The other options involve changes in speed or direction, which do not represent uniform motion.
- For instance, the train and the boy cycling are both affected by their surroundings, causing variations in their speed.
Q13: Which of the following is an example of oscillatory motion?
(a) A boy riding a bicycle on a straight path
(b) Motion of a car or any moving object along a curved line
(c) Pendulum of a clock
(d) Motion of the Earth around the Sun
 View Answer
View AnswerAns: (c)
When an object repeats its motion after every fixed interval of time, the motion of the object is called periodic motion or oscillatory motion. A pendulum of a clock repeats its motion after every fixed interval of time. So, it is an example of oscillatory motion.
Q14: Reeta places two distinct items in each of the following containers. "Container (i): Copper coin and iron nail Container (ii): Steel nail and silver earring Container (iii): Marble and gold coin Container (iv): Nickel coin and steel nail" She can utilize a magnet to distinguish the objects in containers ________.
(a) (i) and (ii)
(b) (i), (ii), and (iii)
(c) (i), (ii), and (iv)
(d) (i), (ii), (iii), and (iv)
 View Answer
View AnswerAns: (a)
- In Container (i), the copper coin is not magnetic, but the iron nail is, so a magnet can separate them.
- In Container (ii), the steel nail is magnetic, while the silver earring is not, allowing separation.
- In Container (iii), neither the marble nor the gold coin is magnetic, so they cannot be separated with a magnet.
- In Container (iv), the nickel coin is not magnetic, but the steel nail is, allowing for separation.
- Thus, a magnet can effectively separate the objects in containers (i) and (ii) only.
Q15: Two boys, X and Y, are running along the same path. X is initially 10 m ahead of Y. However, Y catches up with X after running 60 m. Assuming that both boys are running at a constant speed, what is the ratio of the speeds of X and Y?
(a) 6:5
(b) 7:6
(c) 3:2
(d) 5:3
 View Answer
View AnswerAns: (a)
- Initially, X is 10 m ahead of Y.
- Y runs 60 m to catch up with X, meaning he covers the distance of X's lead plus the distance X runs in that time.
- Let the speed of X be Vx and the speed of Y be Vy. In the time Y runs 60 m, X runs (60 - 10) m = 50 m.
- Thus, the ratio of their speeds is Vx:Vy = 50:60 = 5:6. However, since we need the ratio of Y's speed to X's speed, we flip it to get Vy:Vx = 6:5.
Q16: If we pour cold water over a tin can filled with boiled water, the can gets distorted. This happens because the air inside the can gets
(a) condensed and creates low pressure
(b) condensed and creates high pressure
(c) diluted and creates low pressure
(d) diluted and creates high pressure
 View Answer
View AnswerAns: (a)
When we pour cold water over a tin can filled with boiled water, the can gets distorted because the air inside the can gets condensed and creates low pressure. The higher pressure from outside distorts the shape of the can.
Q17: During the takeoff of an aeroplane, there is a ________ pressure on the top and a _________ pressure below.
(a) low, low
(b) low, high
(c) high, high
(d) high, low
 View Answer
View AnswerAns: (b)
- The pressure on the top of the wings is low during takeoff, which helps the plane to lift.
- Conversely, the pressure below the wings is high, pushing the plane upwards.
- This difference in pressure creates lift, allowing the airplane to ascend into the air.
- Understanding this concept is crucial for grasping how airplanes achieve flight.
Q18: Which of the following options is not correct with regard to laws of reflection?
(a) There are two laws of reflection.
(b) The incident ray, the reflected ray and the normal at the point of incidence lie in the same plane.
(c) Angle of incidence and angle of reflection are same.
(d) The angle between incident ray and reflected ray is less than twice the angle of incident.
 View Answer
View AnswerAns: (d)
The angle between incident ray and reflected ray is equal to the sum of the angle of incident and the angle of reflection. Since both of them are equal, this should be twice the angle of incident, not less than twice.
Q19: Rahul was given a dilute solution of hydrochloric acid and was assigned a task to test it with different indicators. He poured the solution into four different test tubes and labelled them A, B, C and D. He added a few drops of phenolphthalein to test tube A, methyl orange to test tube B, litmus solution to test tube C and red cabbage extract to test tube D.
Which of the following observations made by Rahul is incorrect?
(a) The solution in test tube B turns pink.
(b) The solution in test tube C turns red.
(c) The solution in test tube A turns pink.
(d) The solution in test tube D turns dark pink.
 View Answer
View AnswerAns: (c)
- Indicators are substances that detect the acidic or basic nature of a substance by a change in its colour.
- Phenolphthalein is colourless in neutral and acidic solutions and pink in basic solutions.
Hence, the observation stated in option (c) is incorrect.
Q20: In the table given below, match the changes listed in the first column with their classification mentioned in the second column.
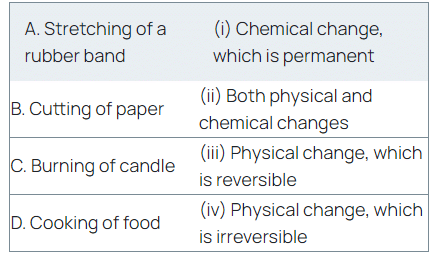
(a) (A) - (ii), (B) - (i), (C) - (iv), (D) - (iii)
(b) (A) - (iii), (B) - (iv), (C) - (ii), (D) - (i)
(c) (A) - (ii), (B) - (iv), (C) - (iii), (D) - (i)
(d) (A) - (iii), (B) - (ii), (C) - (iv), (D) - (i)
 View Answer
View AnswerAns: (b)
- Stretching of a rubber band is a physical change, which is reversible.
- Cutting of paper is a physical change, which is irreversible.
- Burning of wax is a chemical change and melting of wax is a physical change. Therefore, burning of candle involves both physical and chemical changes.
- Cooking of food is a chemical change, which is permanent and irreversible.
Hence, option (b) is correct.
Q21: Which of the following departments measures the elements of weather and keeps their record?
(a) Department of meteorology
(b) Department of agrometeorology
(c) Department of climatology
(d) Research and development department
 View Answer
View AnswerAns: (a)
The department of meteorology is a government department. The meteorological department measures the elements of weather and keeps their record. Meteorologists use data from satellites and analyse the data to forecast about the weather.
Q22: Sheep is an important wool-yielding animal and wool from different breeds of sheep have different applications according to its quality. The options below provide information about the breeds of sheep that are native of different states of India. Select the option in which the information provided is incorrect.
(a) Nali sheep breed is well adapted to the arid and semi-arid regions of Rajasthan and Haryana.
(b) Lohi sheep is found in southern Punjab.
(c) Bhakarwal is a native of Jammu and Kashmir.
(d) Patanwadi, Marwari and Dumba are sheep breeds native of Rajasthan.
 View Answer
View AnswerAns: (d)
Patanwadi, Marwari and Dumba are three important sheep breeds of Gujarat.
Hence, the information in option (d) is incorrect.
Q23: Read the given statements and choose the correct option
Statement 1: Plants and animals are both dependent on each other.
Statement 2: Animals complete the process of nutrient recycling, thus maintain the concentration of soil nutrients.
(a) Both statements 1 and 2 are correct and statement 2 is the correct explanation of statement 1.
(b) Both statements 1 and 2 are correct and statement 2 is not the correct explanation of statement 1.
(c) Both statements 1 and 2 are incorrect.
(d) Statement 1 is correct, but statement 2 is incorrect.
 View Answer
View AnswerAns: (d)
Statement 2 is incorrect. Decomposers, and not animals, complete the process of nutrient recycling, thus maintain the concentration of soil nutrients.
Q24: Which of the following mixtures cannot be separated using the sublimation method?
(a) I only
(b) III and IV only
(c) III only
(d) II only
 View Answer
View AnswerAns: (c)
- The sublimation method is used to separate substances that can change directly from a solid to a gas without becoming liquid.
- In this case, Camphor and Iodine can sublimate, while Common salt and Sugar cannot.
- Thus, mixtures containing Common salt or Sugar cannot be separated by sublimation.
- Among the options, only III (Camphor + Iodine) cannot be separated by sublimation, making it the correct answer.
Q25: While playing football, Harry falls on the ground and his knee starts to bleed. The presence of which of the following will lead to stoppage of the bleeding?
(a) Capillaries
(b) Plasma
(c) Platelets
(d) Red blood cells
 View Answer
View AnswerAns: (c)
Platelets are responsible for clotting or coagulation of blood. In case of an injury, the blood clots after some time. This prevents excess loss of blood. Clotting of blood is a defence mechanism in the body.
Q26: What happens when non-metals combine with oxygen?
(a) Acidic oxide is formed.
(b) Basic oxide is formed.
(c) Amphoteric oxide is formed.
(d) Salts are formed.
 View Answer
View AnswerAns: (a)
Non-metals combine with oxygen to form acidic oxides.
Q27: Metals can be drawn into wires. This property is known as –
(a) Malleability
(b) Lustrous
(c) Ductility
(d) Sonorous
 View Answer
View AnswerAns: (c)
Ductility is the property of a metal associated with the ability to be hammered thin or stretched into wire without breaking.
Q28: Study the given substances.
Substance X reacts with dilute HCI violently. Y reacts slowly while Z does not react at all.
Identify X, Y and Z.
(a) Copper, Aluminium and Iron respectively.
(b) Aluminium, Sodium and Copper respectively.
(c) Sodium, Copper and Iron respectively.
(d) Potassium, Aluminium and Copper respectively.
 View Answer
View AnswerAns: (d)
More reactive metals like sodium and potassium react violently with dilute acids. Reaction is slower for less reactive metals and, copper, silver and gold do not react with acids.
Q29: Four experiment on rusting are shown below :
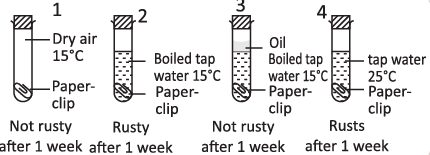
Which two experimental set-up can be used to show that air is needed for iron to rust?
(a) 1 & 3
(b) 1 & 4
(c) 2 & 3
(d) 2 & 4
 View Answer
View AnswerAns: (d)
Iron reacts with moist air to form rust (Fe2O3 · xH2O). Since, in test tube 1, air is dry and test tube 3 has a layer of oil on the top of water, so iron (paper clip) does not come in the direct contact of moist air, and does not get rust.
Q30: Select the incorrect match.
(a) Bakharwal - Jammu and Kashmir
(b) Nali - Haryana
(c) Lohi - Uttar Pradesh
(d) Patanwadi - Gujarat
 View Answer
View AnswerAns: (c)
- Lohi is actually a breed associated with Haryana, not Uttar Pradesh. This makes option (c) the incorrect match.
- The other options correctly match the breeds with their respective regions: Bakharwal is from Jammu and Kashmir, Nali is from Haryana, and Patanwadi is from Gujarat.
- Understanding the geographical distribution of these breeds is important for recognizing their origins.
Q31: Read the given statements and select the option that correctly fills the blanks in any two of them.
(i) _______ are the tiny outgrowths on the inner surface of the small intestine.
(ii) Mucus, hydrochloric acid, and digestive juice are secreted by ________.
(iii) The length of the small intestine in an adult human being is about ________.
(iv) _______ mainly absorbs water from the undigested food.
(a) (i) Villi (ii) Stomach
(b) (iii) 5 m (iv) Small intestine
(c) (i) Buds (iii) 7 m
(d) (ii) Small intestine (iv) Large intestine
 View Answer
View AnswerAns: (a)
- Villi are the small, finger-like projections found on the inner surface of the small intestine, which help in nutrient absorption.
- The stomach is responsible for secreting mucus, hydrochloric acid, and digestive juices that aid in digestion.
- The other options do not correctly fill the blanks as they either provide incorrect terms or measurements.
- Thus, option (a) is the correct choice as it accurately completes the statements.
Q32: Which of the following statements about polar bears' adaptations to cold environments is/are incorrect?
(i) White fur makes it difficult for prey and predator to see polar bear in snow.
(ii) A single thick layer of fur helps to keep polar bear warm.
(iii) Wide and large paws make it easier for polar bear to walk on snow.
(iv) Strong sense of smell helps polar bear to catch its prey.
(a) (i) only
(b) (i) and (iv) only
(c) (ii) and (iii) only
(d) (ii) only
 View Answer
View AnswerAns: (d)
- The statement (i) is correct because the white fur of polar bears actually helps them blend into the snow, making it hard for both prey and predators to spot them.
- Statement (ii) is incorrect; polar bears have two layers of fur: a dense undercoat and a longer outer layer, which together keep them warm.
- Statement (iii) is correct; their large paws distribute their weight, allowing them to walk on snow without sinking.
- Statement (iv) is correct; polar bears have a strong sense of smell that aids them in locating prey.
Q33: Saloni took a piece of burning charcoal and collected the gas evolved in a test tube. As a confirmatory test, she passed the gas through a test tube filled with lime water and observed that the lime water turned milky. Which of the following was the gas evolved?
(a) Oxygen
(b) Nitrogen
(c) Carbon dioxide
(d) Hydrogen
 View Answer
View AnswerAns: (c)
The test confirms that the gas was carbon dioxide. When passed through lime water it turns it milky because of the formation of calcium carbonate, which is a white compound insoluble in water.
The following reaction occurs:
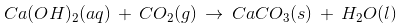
Q34: Which of the following methods would be useful to tackle the shortage of water?
(a) Rainwater harvesting
(b) Drip irrigation
(c) Roof irrigation
(d) All of the above
 View Answer
View AnswerAns: (d)
- Collection of rainwater for future use is called rainwater harvesting.
- Drip irrigation is a method through which maximum number of plants can be irrigated with a minimum quantity of water. For this, pipelines are laid throughout the rows of plants with holes at strategic points to release water.
- In roof irrigation, water is gathered on the roof for irrigation.
- All these methods help in saving water, which is an important but scarce resource.
Q35: 19. Select the correct statement.
(a) Metals have fixed melting points whereas non-metals do not.
(b) All metals are malleable.
(c) The hardest substance known is a non-metal.
(d) Tungsten is a bad conductor of heat and electricity.
 View Answer
View AnswerAns: (c)
The hardest substance known is diamond. Diamond is an allotrope of carbon, i.e., a non-metal.
Q36: Which of the following traits is not true about flowers that are pollinated by wind?
(a) Huge quantities of pollen grains are produced.
(b) Petals are bright in color.
(c) Pollen grains are light and non-sticky.
(d) Well exposed stamens and stigma.
 View Answer
View AnswerAns: (b)
- The correct answer is (b) because flowers that rely on wind for pollination typically do not have bright petals. Instead, they often have small or no petals to avoid attracting insects.
- These flowers produce huge quantities of pollen grains to increase the chances of successful pollination.
- Pollen grains from wind-pollinated flowers are usually light and non-sticky, allowing them to be carried easily by the wind.
- Additionally, they have well exposed stamens and stigma to maximize the chances of catching the wind-borne pollen.
Q37: In an experiment, Vihaan kept a solution of copper sulphate in an iron container for a few days. What is the reason for your observation?
(a) The surface of iron container will develop a green layer due to corrosion caused by copper sulphate.
(b) Copper metal will be deposited in iron container since copper is more reactive than iron.
(c) Nothing will happen since no reaction takes place between copper sulphate and iron.
(d) Holes will be formed in the container since iron is more reactive than copper hence iron displaces copper from a solution of copper sulphate.
 View Answer
View AnswerAns: (b)
As the solution turns blue litmus red so, it is an acidic solution. On burning non-metals, it forms acidic oxides. Therefore, the substance which Rahul burned must be a non-metal. Since, Mg, Na and Fe are metals while S is a non-metal, so the burning substance is sulphur (S).
Q38: In an experiment, Rahul took a burning substance and collected the evolved vapours in a test tube, making sure that they do not escape. Then he added a small amount of water to the test tube. To the solution obtained, a few drops of blue litmus solution was added. This turned the litmus solution red. Identify the burning substance?
(a) Magnesium (Mg)
(b) Sulphur (S)
(c) Sodium (Na)
(d) Iron (Fe)
 View Answer
View AnswerAns: (a)
Chalk powder and water can be separated by sedimentation and decantation while salt and water can be separated by evaporation.
Q39: A science teacher organised a quiz in the class. He stated few applications of non-metals and asked students to guess the names of the non-metals. Mark the correct option.
(a) Non-metals used in fertilisers - Potassium and Chlorine
(b) Non-metal used in water purification process - Bromine.
(c) Non-metal used in crackers - Nitrogen
(d) Non-metal used as an antiseptic - Iodine
 View Answer
View AnswerAns: (d)
Non-metals used in fertilisers are nitrogen and phosphorus; non-metal used in water purification process is chlorine ; non- metals used in crackers are sulphur and phosphorus.
Q40: Why are dark and woollen clothes preferred in winter?
(a) Because dark clothes absorb less heat and wool is a good conductor of heat
(b) Because dark clothes absorb more heat and wool is a good conductor of heat
(c) Because dark clothes absorb more heat and wool is a bad conductor of heat
(d) Because dark clothes and wool are both warm
 View Answer
View AnswerAns: (c)
Dark clothes absorb more heat and keep one comfortable in winter. Woollen clothes are used in winter season because wool is a poor conductor of heat. In addition to it, air gets trapped in woollen fibre to further increase the poor conductivity of wool. This prevents the radiation of heat of our body to the surrounding and prevents the cold from surroundings to affect our body.
Q41: Select the incorrect pairing of an animal with its adaptive characteristic.
(a) Snow leopard - Thick fur
(b) Dolphin - Blow holes to breathe
(c) Deer - Long ears to hear movement of predators
(d) Camel - Produces a large volume of urine to manage body temperature in warm conditions
 View Answer
View AnswerAns: (d)
- The question asks for the incorrect match between an animal and its adaptive feature.
- Snow leopards have thick fur to keep warm in cold climates, which is correct.
- Dolphins use blow holes to breathe, which is also accurate.
- Deer have long ears to detect predators, making this pairing correct as well.
- However, camels do not excrete a large amount of urine; instead, they conserve water and can tolerate dehydration, making option (d) the incorrect match.
Q42: The different steps of the water cycle are given below randomly.
Select the option that shows the correct sequence of these steps.
(i) Water droplets in clouds join together to form large droplets.
(ii) Water vapor from water bodies and from leaves of plants get added to air.
(iii) Water vapor gets cooled at height.
(iv) Rainwater goes back to the water bodies.
(v) Drops of water fall on the Earth as rain.
(vi) Water droplets form a cloud.
(a) (ii) → (vi) → (iii) → (i) → (v) → (iv)
(b) (iii) → (ii) → (vi) → (i) → (v) → (iv)
(c) (ii) → (iii) → (vi) → (i) → (iv) → (v)
(d) (ii) → (iii) → (vi) → (i) → (v) → (iv)
 View Answer
View AnswerAns: (d)
- Step 1: Water vapor from water bodies and plants enters the air (ii).
- Step 2: This vapor cools at higher altitudes (iii).
- Step 3: The cooled vapor forms clouds (vi).
- Step 4: Water droplets in clouds combine to create larger droplets (i).
- Step 5: These droplets fall as rain (v).
- Step 6: Finally, the rainwater returns to water bodies (iv).
This sequence accurately represents the water cycle, showing how water moves from the ground to the atmosphere and back again.
Q43: Air _______ with the increase in altitude.
(a) Gets thinner
(b) Gets thicker
(c) Remains same in thickness
(d) Cannot change its composition
 View Answer
View AnswerAns: (a)
- Air becomes thinner as you go higher in altitude. This means that there are fewer air molecules in a given volume.
- As you climb mountains or fly in an airplane, you may notice that it becomes harder to breathe; this is because the oxygen level decreases with altitude.
- The thinning of air affects various activities, such as sports and aviation, where less air pressure can impact performance.
- In summary, as altitude increases, the air density decreases, making it thinner.
Q44: Fast moving air around a centre, usually with heavy rain, is known as a cyclone. Wind direction, wind speed, humidity and temperature together create cyclone. What is the eye of a cyclone?
(a) Calm area of the cyclone
(b) The centre of the cyclone
(c) High speed area of the cyclone
(d) Both (1) and (2)
 View Answer
View AnswerAns: (a)
The centre of the cyclone is called the 'eye'. A cyclone may be 10 km to 15 km high. It is a rotating mass of air. The diameter of the cyclone eye may vary from 10 km to 30 km. The eye of a cyclone is the calm area, but around the eye, air may move with high speeds such as 150 km/h to 250 km/h.
Q45: Select the group that contains complete flowers.
(a) Petunia, Hibiscus, Mustard
(b) Pea, Rose, Papaya
(c) Date palm, Gulmohar, Mulberry
(d) Mulberry, Papaya, Pea
 View Answer
View AnswerAns: (a)
- Complete flowers have all four main parts: sepals, petals, stamens, and carpels.
- In option (a), Petunia, Hibiscus, and Mustard all possess these four parts, making them complete flowers.
- Options (b), (c), and (d) include plants that either lack some parts or are incomplete flowers.
- Thus, the correct answer is (a) as it includes only complete flowers.
Achievers Section
Q46: Analyse the two statements given below and select the correct option.Statement I: Sericulture is the term used for rearing of Bombyx mori silkworms for the production of mulberry silk.
Statement II: Moriculture is the term used for rearing of silkworms that belong to the genus Antheraea for the production of tasar silk.
(a) Both statements I and II are correct and statement II is the correct reason for I.
(b) Both statements I and II are correct, but statement II is not the correct reason for I.
(c) Statement I is correct, but statement II is incorrect.
(d) Both statements I and II are incorrect.
 View Answer
View AnswerAns: (d)
- Both sericulture and moriculture are activities related to production of silk.
- Sericulture is the general term used for rearing of silkworms for commercial production of silk.
- Moriculture is cultivation of mulberry because the silkworm Bombyx mori feeds on mulberry leaves.
Therefore, both statements I and II are incorrect.
Q47: Aluminium is used for making cooking utensils. Which of the following properties of Aluminium is responsible for the same.
I. Thermal conductivity
II. Good electrical conductivity
III. Ductility IV. High melting point
(a) I and II only
(b) I and III only
(c) II and III only
(d) I and IV only
 View Answer
View AnswerAns: (d)
Aluminium is used for making cooking utensils because it has high melting point and good thermal conductivity.
Q48: Which of the following is NOT used for electroplating metal articles?
(a) Nickel
(b) Silver
(c) Chromium
(d) Sodium
 View Answer
View AnswerAns: (d)
Nickel, silver and chromium are active electrodes so they can be used for electroplating the articles but sodium is not used for electroplating.
Q49: Read the given paragraph where a few words have been italicized.
Wastewater treatment plant involves physical, chemical, and biological processes. First, the wastewater is made to pass through bar screens where liquid materials are removed. Then water is allowed to go into the grit tank where the speed of wastewater is increased so that light objects settle at the bottom. Then water enters the sedimentation tank where sludge sinks slowly at the bottom, which is continuously removed by a skimmer. Water then enters the aeration tank which contains anaerobic bacteria. Select the correct statement regarding this.
(a) Bar screen should be replaced by digester and liquid should be replaced by solid.
(b) The positions of grit and aeration should be interchanged.
(c) Increased and light should not be replaced as they are correctly mentioned.
(d) Skimmer should be replaced by scraper and anaerobic should be replaced by aerobic.
 View Answer
View AnswerAns: (d)
- Skimmer is a device used to remove sludge, and it can be correctly replaced by a scraper in this context.
- The term anaerobic refers to bacteria that do not require oxygen, while aerobic refers to those that do. In the aeration tank, aerobic bacteria are typically used.
- Options (a) and (b) suggest incorrect replacements that do not align with the processes described.
- Option (c) is correct in stating that "increased" and "light" are appropriately used, but it does not address the main focus of the question.
Q50: Which of the following options is FALSE?
(a) A kind of plant grown and cultivated on a large scale at a place is called crop.
(b) The first step before growing crops is preparation of the soil.
(c) Damaged seeds would sink in the water.
(d) For growing a crop, sufficient sunlight, water and nutrients from the soil are essential.
 View Answer
View AnswerAns: (c)
A kind of plant grown and cultivated on a large scale at a place is called crop. The first step before growing crops is preparation of the soil. For growing a crop, sufficient sunlight, water and nutrients from the soil are essential. Damaged seeds would float on top of the water.
|
34 videos|88 docs|54 tests
|
FAQs on Science Olympiad Model Test Paper - 2 - Science Olympiad Class 7
| 1. What topics are covered in the Science Olympiad Model Test Paper - 2 for Class 7? |  |
| 2. How can students prepare effectively for the Science Olympiad? |  |
| 3. What is the format of the questions in the Science Olympiad Model Test Paper - 2? |  |
| 4. How is the Science Olympiad beneficial for Class 7 students? |  |
| 5. What resources are recommended for preparing for the Science Olympiad? |  |






















