Short & Long Question Answers: Isolation of Elements | Chemistry for JEE Main & Advanced PDF Download
Q.1 What is slag?
Ans. It is easily fusible material fusible material, which is formed when gangue still
present in roasted ore combines with the flux.
e.g. CaO (flux) + SiO2 (gangue) →CaSiO3 (slag)
Q.2 Which is better reducing agent at 983K, carbon or CO?
Ans. CO, (above 983K CO being more stable & does not act as a good reducing agent but
carbon does.)
Q.3 At which temperature carbon can be used as a reducing agent for Foe?
Ans. Above 1123 K, carbon can reduce FeO to Fe.
Q.4 What is the role of graphite rods in electrometallurgy of aluminum?
Ans. Graphite rods act as anode, are attacked by oxygen to form CO2 and so to be replace
time to time.
Q.5 What is the role of cryolite in electrometallurgy of aluminum?
Ans. alumina cannot be fused easily because of high melting point. Dissolving of alumina
in cryolite furnishes Al3+ ions, which can be electrolyzed easily.
Q.6 What are depressants?
Ans. It is possible to separate two sulphide ore by adjusting proportion of oil to water in
froth flotation process by using a substance known as depressant. e.g. NaCN is used to
separate ZnS and PbS.
Q.7 Copper can be extracted by hydrometallurgy but not Zn. Why?
Ans. The E0 of Zn is lower than that of Cu thus Zn can displace Cu2+ ion from its solution.
On other hand side to displace Zn from Zn2+ ion, we need a more reactive metal than it.
Q.8 Give name and formula of important ore of iron.
Ans. Haematite - Fe2O3 , Magnetite -Fe3O4 , Iron pyrites FeS2 .
Q.9 Give name and formula of important ore of Copper.
Ans. Copper pyrites CuFeS2 , Malachite CuCO3 . Cu (OH) 2 , Cuprite Cu2O.
Q.10 Give name and formula of important ore of Zinc.
Ans. Zinc blende - ZnS, Calamine- ZnCO3 , Zincite - ZnO
Q.11 Describe the method of refining of nickel.
Ans. In the Mond Process, Ni is heated in a stream of CO forming a volatile complex, which then decomposes at higher temperature to give Ni.
At 330-350K: - Ni + 4CO → Ni (CO) 4
At 450-470K Ni (CO)4 → Ni + 4 CO
Q.12 What is Zone Refining? Explain with example.
Ans. Zone refining is a method of obtaining a metal in very pure state. It is based on the
principal that impurities are more soluble in molten state of metal than solidified state.
In this method, a rod of impure metal is moved slowly over circular heater. The portion
of the metal being heated melts & forms the molten zone. As this portion of the rod moves
out of heater, it solidified while the impurities pass into molten zone. The process is
repeated to obtain ultrapure metal and end of rod containing impure metal cutoff.
Q.13 Write the principal of electro-refining.
Ans. In this method of purification impure metal is made Anode and pure metal is made the cathode. On passing electricity, pure metal is deposited at the cathode while the impurities dissolve dissolve in solution as anode mud. E.g. electro- refining of copper:-
At Cathode: - Cu 2+ + 2e → Cu
At Anode: - Cu → Cu 2+ + 2e
Q.14 Write difference between calcinations and roasting.
Ans. Roasting: It is used to convert sulphide ores into oxides. Roasting involves strong
heating of iron ore in the presence of excess air. For example, copper sulphide in copper
glance ore is converted into copper (I) oxide by heating it in the presence of oxygen.
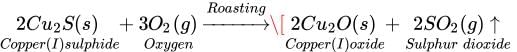
Calcination: It is used to convert carbonate ores into oxides. Calcination involves strong
heating of the ore in the absence of air. For example, calamine ore, which is chemically
zinc carbonate, is converted into zinc oxide by heating it in the absence of air.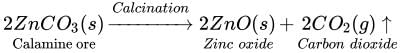
Q.15 Describe the method of refining of Zirconium and Titanium.
Ans. Van Arkel process is used for obtaining ultrapure metal. The impure metal is converted into volatile compound, which then decomposes electrically to get pure metal. At 850K: - Zr impure) + 2 I2 → ZnI4
At 2075K:- ZnI4 → Zr (pure) + 2 I2
Q.16 Out of C & CO, which is better reducing agent for ZnO?
Ans. Since free energy of formation of CO from C is lower at temperature above 1120K
while that of CO2 from carbon is lower above 1323K than free energy of formation of
ZnO. However, the free energy of formation of CO2 from CO is always higher than that of
ZnO. Hence, C is better reducing agent of ZnO.
Q.17 The value of ∆fG0 for Cr2O3 is -540kJ/mole & that of Al2O3 is -827kJ/mole. Is the reduction of Cr2O3 possible with aluminium?
Ans. The desired conversion is
4 Al + 2Cr2O3→2Al2O3 + 4Cr
It is obtained by addition of following two reactions:-
4Al + 3O2→ 2 Al2O3 ∆f G0=-827kJ/mole
2Cr2O3 → 4Cr + 3O2 ∆f G0==+ 540 kJ/mole
Therefore, A G0 for desired reaction is -827+540=-287, as a result reduction is possible.
Q.18 Why copper matte is put in silica lined converter?
Ans. Copper matte consists of Cu2S and FeS. When blast of air is passed through molten
matte in silica- lined converter, FeS present in matte is oxidized to FeO, which combines
with silica to form slag.
I. 2FeS + 3O2→2FeO +2 SO2,
II. FeO + SiO2→FeSiO3(slag),
III. 2Cu2S + 3O2 →2Cu2O+2SO2,
IV. 2Cu2O+2Cu2S→6Cu + SO2
Q.19 What is meant by term chromatography?
Ans. Chromato means Colour and graphy means writing because the method was first
used for separation of coloured substance. It is based on selective distribution of various
constituents of a mixture between two phases, a stationary phase and a moving phase. The stationary phase can be either solid or liquid on solid support.
Q.20 Why is reduction of metal oxide easier if metal formed is in liquid state at temperature of reduction.
Ans. The entropy of a substance is higher in liquid state than solid state. In the reduction
of metal oxide, the entropy change will be positive if metal formed is in liquid state. Thus,
the value of A G0 becomes negative and reduction occurs easily.
SHORT ANSWER TYPE QUESTION
Q.1. Explain the following:-
i. Zinc but not copper is used for recovery of Ag from the complex [Ag(CN)2].
ii. Partial roasting of sulphide ore is done in the metallurgy of copper.
iii. Extraction of Cu from pyrites is difficult than that from its oxide ore through reduction.
Ans. a. A.1- (i) Zn is more powerful reducing agent in comparison to copper. Zn is also
cheaper than Cu.
b. Partial roasting of sulphide ore forms some oxide. This oxide then reacts with
remaining sulphide ore to give copper i.e. self-reduction occurs.
2Cu2S + 3O2 →2Cu2O+2SO2,
2Cu2O+2Cu2S→6Cu + SO2.
c. Though carbon is good reducing agent for oxide but it is poor reducing agent for
sulphides. The reduction of metal sulphide does not have large negative value.
Q.2 Explain the method for obtaining pig iron from magnetite.
Ans. Extraction of iron from Magnetite takes place in following steps:-
I. Concentration of ore: - It is done by Gravity separation followed by magnetic separation process.
II. Calcination: - It involve heating when the volatile matter escapes leaving behind metal oxide. Fe2O3 .xH2O→ Fe2O3 + xH2O.
III. Roasting: - It involves heating of ore in presence of air, thus moisture, CO2 ,SO2 , As2O3 removed And FeO oxidized to Fe2O3 .
IV. Smelting of roasted ore: - A mixture of ore, coke & CaCO3 is smelted in long BLAST FURNACE. Following reaction takes place at different temperature zones:-
i. Zone of reduction: - Temperature range 250°C-700°C
3Fe2O3+CO → 2Fe3O4+CO2
Fe3O4+CO → 3FeO+ C02
FeO +CO → 1 Fe+ C02
ii. Zone of slag formation:- Temperature range 800°C-1000°C
CaCO3 → CaO+C02
CaO+SiO2 → CaSiO3 , P4O10+IOC 4P+10CO,
SiO2+2C → Si+2CO, MnO2+2CF Mn+2CO
iii. Zone of fusion:- Temperature range 1150°C-1350°C
CO2+C → 2CO
iv. Zone of fusion:- Temperature range 1450°C-1950°C
C+O2 → CO2
Thus, Pig iron is obtained from Blast Furnace.
Q.3 Name the principal ore of aluminum and describe how Al is extracted from its ore.
Ans. Important ores -(i) Bauxite Al2O3 .xH20 (ii) Corundum Al2O3 . Bauxite is commercially important ore Al.
Extraction from Bauxite ore involves the following two stages:-
i. Purification of bauxite to get pure alumina (Al2O)
ii. Electrolysis of pure alumina in molten cryolite
Step:-1 Bauxite is treated with NaOH .Following reaction takes place:-
Al2O3 +2NaOH + 3 H20 2Na [Al(OH)4 ] and impurities of Fe2O3 ,TiO2 & SiO2 are removed . Na [Al(OH)4 ] ,then reacts with CO2 then pure Alumina is obtained.
Na [Al(OH)4 ] + 2CO2 → Al2O3 .xH2O + 2NaHCO3
Step:-2 Electrolytic reduction of pure alumina takes place in iron box (cathode) with cryolite (Na3AlF6 ) & fluorspar CaF2 .Graphite rods act as anode. Following reactions take place:- At cathode:- Al 3+ + 3e→ Al, At Anode:- 2O2-→O2 + By this process 98.8% pure Aluminum is obtained.
Q.4 Describe the principles of extraction of Zinc from zinc blende.
Ans. Important ores of Zn:-Zinc blende - ZnS, Calamine- ZnCO3 , and Zincite - ZnO. ZnS is commercially important ore of Zn.Various stages involved in the extraction of Zn from
ZnS are as following:-
I. Concentration of ore:-It is concentrated by Froth flotation process followed by gravity
separation process.
II. Roasting:- The concentrated ore is roasted in presence of air. Following reactions take
place:-
2ZnS + 3O2 →2ZnO + 2SO2
The mass obtained during roasting is porous and is called porous clinker.
III. Reduction of ZnO to Zn: - ZnO is made into briquettes with coke and clay and heated at 1163K. Zn formed distills off and is collected by rapid cooling of zinc vapours.
ZnO + C → Zn + CO
|
334 videos|660 docs|300 tests
|
FAQs on Short & Long Question Answers: Isolation of Elements - Chemistry for JEE Main & Advanced
| 1. What is isolation of elements in the context of the JEE exam? |  |
| 2. What are some commonly used methods for isolating elements? |  |
| 3. How is electrolysis used in the isolation of elements? |  |
| 4. Can you explain the concept of reduction in the isolation of elements? |  |
| 5. What is fractional distillation and how is it employed in the isolation of elements? |  |
















