Very Short Answer Type Questions
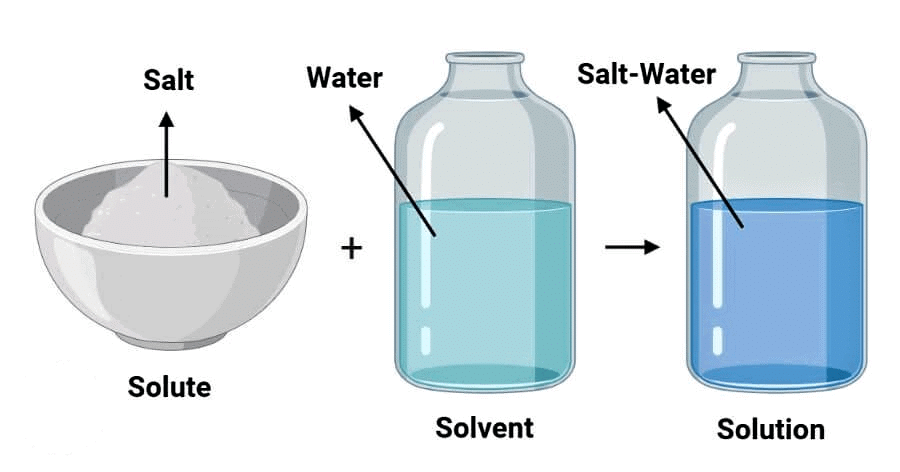
Q1. Define solvent.
A solvent is the substance in a solution that dissolves the solute. Example: In a saltwater solution, water is the solvent.
Q2. Define solute.
A solute is the substance that gets dissolved in a solvent to form a solution. Example: In a saltwater solution, salt is the solute.
Q3. What is ‘tincture of iodine’?
A solution of iodine in alcohol is known as tincture of iodine. It has iodine (solid) as the solute and alcohol (liquid) as the solvent.
Q4. What are alloys?
Alloys are mixtures of two or more metals, or a metal and a non-metal. They cannot be separated into their individual components through physical methods.
Example: Steel is an alloy of iron and carbon.
Q5. Give one example of a gas in a liquid solution.
In cold drinks, carbon dioxide gas is the solute and water is the solvent.
Q6. How can a solution be dilute or concentrated?
A solution is dilute if it contains a small amount of solute in the solvent, and concentrated if it contains a large amount of solute in the solvent.
Q7. What is “concentration of a solution”?
The concentration of a solution refers to the amount of solute present in a given amount of solvent or solution. It indicates how concentrated or dilute a solution is.
Q8. State the difference between aqueous and non-aqueous solutions.
Aqueous solutions use water as the solvent, while non-aqueous solutions do not contain water as the solvent.
Q9. What is the “solubility” of a solute?
The amount of a solute that can dissolve in a given amount of solvent at a specific temperature is known as its solubility.
Q10. What is a saturated solution?
A saturated solution is one in which the maximum amount of solute is dissolved in a solvent at a given temperature, and no more solute can dissolve further.
Q11. What is an unsaturated solution?
An unsaturated solution contains less solute than the maximum amount it can dissolve at a given temperature.
Q12. How can you convert the saturated solution into an unsaturated one or vice versa?
A saturated solution can be converted to an unsaturated solution by:
To convert a saturated solution into an unsaturated one, heat the solution so more solute can dissolve.
To convert an unsaturated solution into a saturated one, cool the solution or add more solute until no more dissolves.
Q13. Why is water called a universal solvent?
Water is called a universal solvent because:
- It can dissolve a wide variety of substances due to its polar nature, which allows different molecules to interact with it effectively.
Example: In our body, water dissolves salts, sugars, and gases to help with digestion, transport, and cell functions.
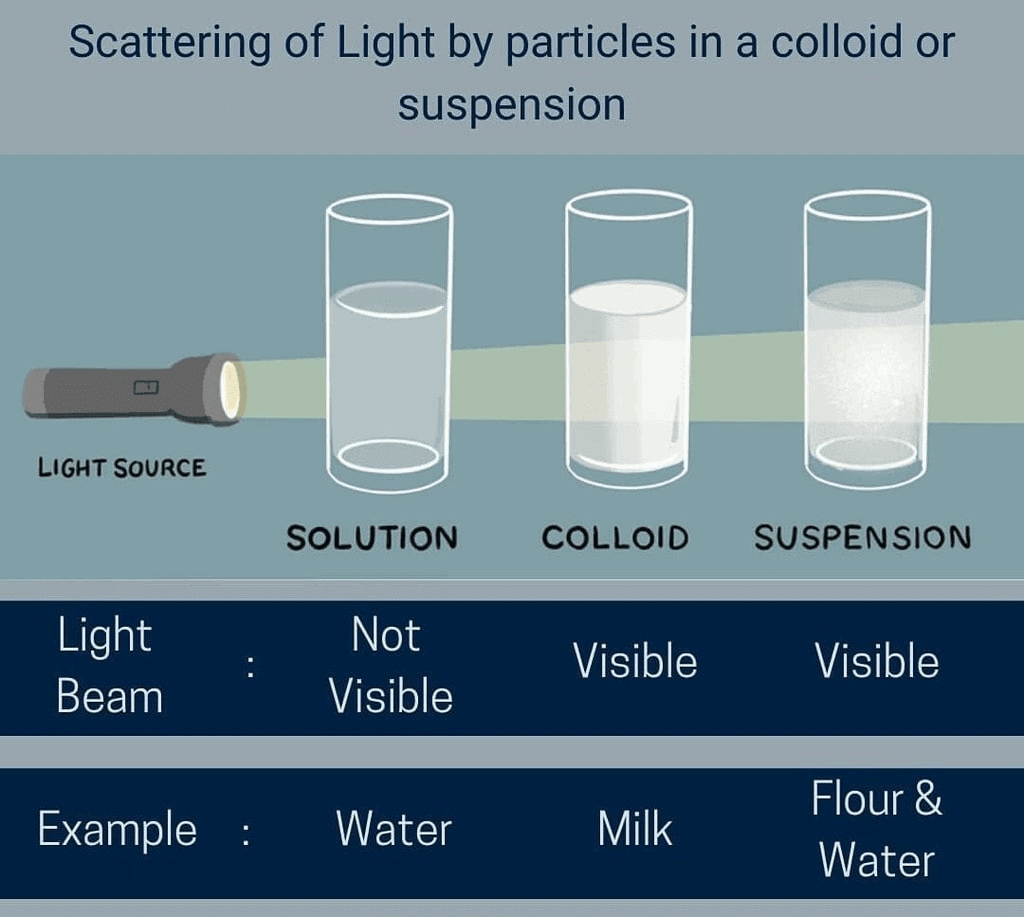
Q14. What is the Tyndall effect?
The scattering of light by colloidal particles is known as Tyndall effect.
Q15. How can we separate colloidal mixtures?
Colloidal mixtures can be separated using centrifugation. This process involves:
- Placing the colloidal solution in a test tube.
- Rotating the test tube rapidly in a centrifuge machine.
- Utilising centrifugal force to separate the colloidal particles from the mixture.
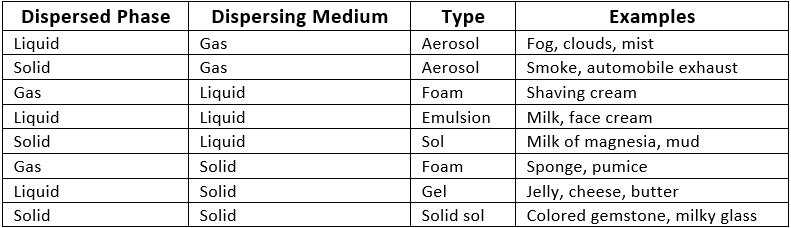
Q16. What is an emulsion?
An emulsion is a mixture where both the dispersed phase and the dispersing medium are liquids.
Example: Milk, Face Cream.
Q17. What is aerosol?
An aerosol is a mixture where solid or liquid particles are dispersed in a gas.
Example: Smoke, Fog.
Q18. What is the principle for the separation of immiscible liquids?
The principle of separation of immiscible liquids is based on their density differences. The less dense liquid collects at the top, and the denser one settles at the bottom.
Q19. How can you separate two liquids that have less than 25 K difference of boiling points?
To separate a mixture of two or more miscible liquids with a boiling point difference of less than 25 K, the method used is fractional distillation.
Q20. What is crystallisation?
When a saturated solution is heated and then allowed to cool slowly, crystals of the dissolved solute separate out. This process, called crystallisation, is used to purify solids.
Short Answer Type Questions
Q1. Why is a mixture called an impure substance?
A mixture is called an impure substance because it contains different components that:
- Retain their individual properties
- Can be easily separated by physical processes
Unlike pure substances, which have a uniform composition, mixtures consist of two or more pure substances combined together.
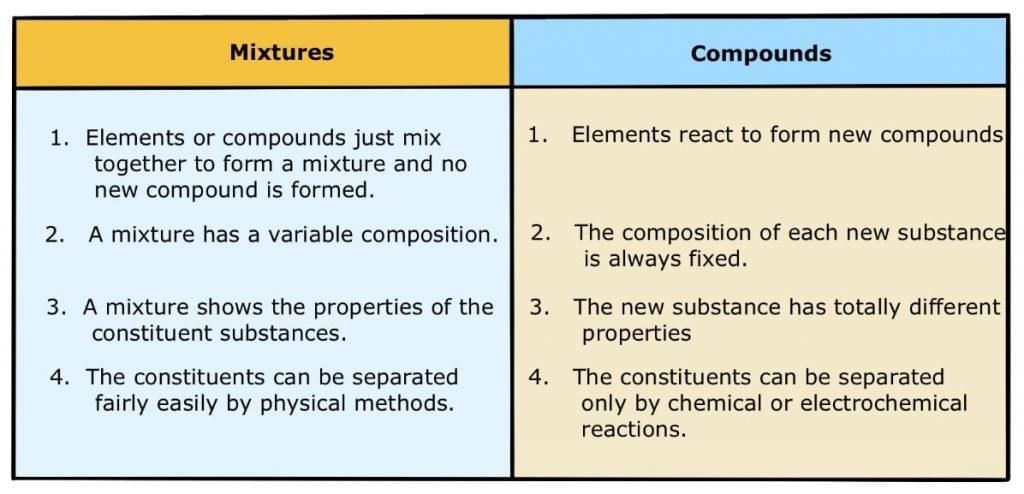
Q2. Give the differences between a mixture and a compound.
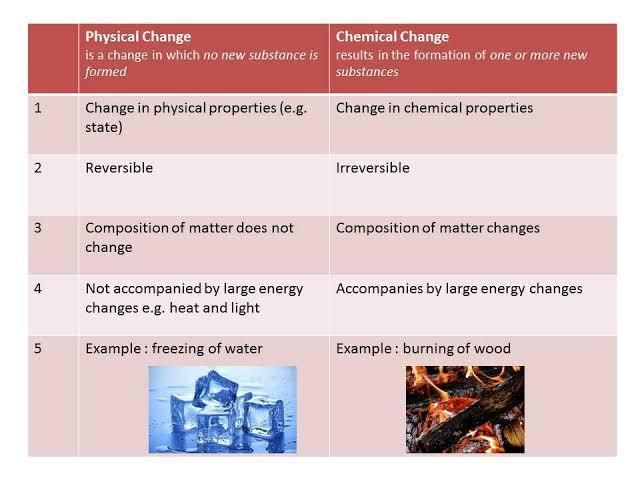
Q3. Distinguish between a physical change and a chemical change.
Q4. State the properties of a solution.
Properties of a solution are:
- A solution is a homogeneous mixture.
- Particles in a solution are smaller than 1 nm and are invisible to the naked eye.
- They do not scatter a beam of light, making the path of light invisible.
- Solute particles cannot be separated by filtration, indicating that a solution is stable.
Q5. State the properties of a suspension.
Properties of a Suspension:
- Heterogeneous mixture: A suspension consists of different components that are not uniformly mixed.
- Visible particles: The particles in a suspension can be seen with the naked eye.
- Tyndall effect: When light passes through a suspension, it scatters, making the path of the light visible.
- Unstable: The particles will eventually settle at the bottom if left undisturbed, indicating that a suspension is not stable.
- Separation: The components can be separated using filtration.
Q6. What is a colloidal solution?
A colloidal solution is a type of heterogeneous mixture that looks homogeneous. Its particles are very small, typically less than 1 micrometre, making them invisible to the naked eye. However, these particles remain suspended and do not settle.
Example: Milk and Blood.
Q7. State the properties of a colloidal solution.
Properties of colloidal solution:
- A colloidal solution is a heterogeneous mixture with particle sizes ranging from 1 nm to 100 nm.
- The particles are so small that they cannot be seen with the naked eye.
- Colloidal solutions can scatter light, which is known as the Tyndall effect.
- They are stable because the particles do not settle when undisturbed.
Q8. Give the applications of centrifugation.
Applications of centrifugation are:
- Centrifugation is used in diagnostic laboratories for blood and urine tests.
- It is applied in dairies and at home to separate butter from cream.
- It is also used in washing machines to remove water from wet clothes.
Q9. Why is crystallisation better than evaporation?
Crystallisation is a process that separates a pure solid in the form of its crystals from a solution. It is often preferred over evaporation for the following reasons:
- During evaporation, some solids may decompose or, like sugar, become charred when heated to dryness.
- Some impurities can remain dissolved in the solution, which may contaminate the solid after evaporation.
Q10. A student is given a mixture of naphthalene balls’ powder and common salt. He needs to separate this mixture. How will he do this?
- The properties of both naphthalene and common salt should be known before we choose the separation technique.
- Naphthalene is a sublimate which on heating changes to gaseous state directly. Hence to separate a volatile compound (sublimate) from a non-volatile compound (non-sublimate), the sublimation process is used.
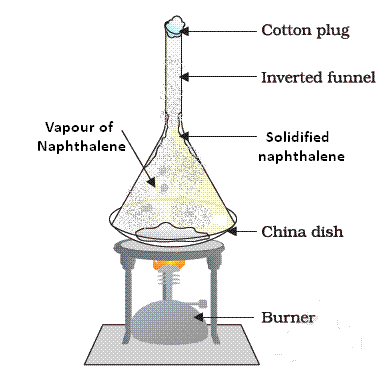
Sublimation of Naphthalene
- In a China dish, the mixture is kept and is placed on a stand. An inverted funnel is kept over the mixture in China dish with plugged stem. The sublimate on heating gets collected on the funnel and common salt remains in the China dish.
Q11. How can we obtain different gases from the air?
Air is a mixture of gases, and we can separate its components using fractional distillation. This process involves:
- Cooling air to a liquid state.
- Gradually warming the liquid to allow different gases to evaporate at their specific boiling points.
- Collecting the gases separately.
Below is a flow diagram illustrating the steps involved in this process:
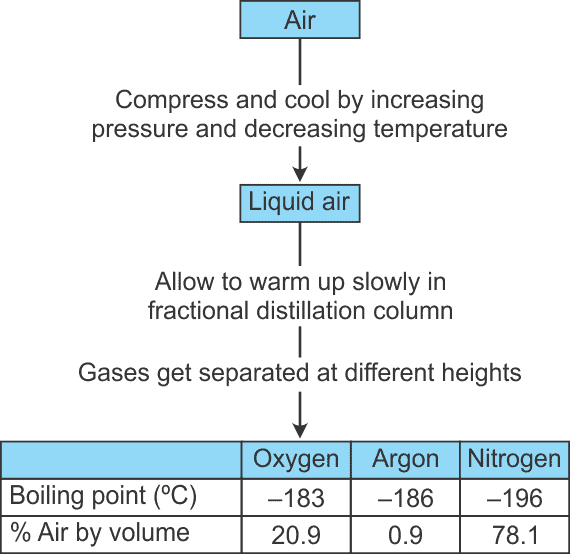
Q12. Draw a flow diagram to show the water purification system in waterworks.
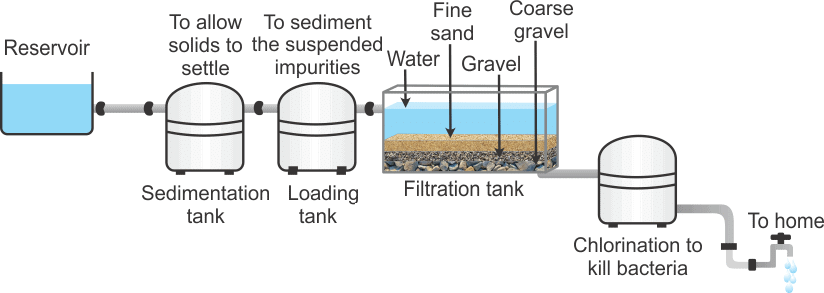
The water purification process involves several key steps:- Collection: Water is collected in a reservoir.
- Sedimentation: Water is sent to a sedimentation tank where solids settle at the bottom.
- Loading Tank: The water moves to a loading tank, allowing suspended impurities to settle as sediment.
- Filtration: Water passes through a filtration tank, where it goes through layers of sand and gravel to remove impurities.
- Chlorination: The clear water is mixed with chlorine or bleaching powder in a chlorinated tank to kill bacteria.
- Distribution: Finally, the purified water is supplied to homes.
Q13. Why is air considered a mixture and not a compound?
Air is considered as a mixture because it exhibits following properties:
1. Each component present in air retains its properties.
2. Each component can be separated by simple physical processes.
3. The components do not have any fixed proportion. All gases are present in different amount.
Example: In greener area—more oxygen and water vapour is present; near industrial area—air consists of a lot of impurities and smoke suspended in it.
Q14. How can you prove that water is a compound?
Water is a compound because if we pass electricity through it then at two different electrodes, we get two different gases i.e., oxygen and hydrogen during the electrolysis of water. The ratio of oxygen: hydrogen is 1: 2 by number of molecules.
(i) The properties of oxygen and hydrogen gases are entirely different from liquid water.
(ii) The ratio of oxygen: hydrogen combination is always constant i.e., 1: 2 by volume.
(iii) To separate the components of water, we need electrolytic cell, and it is not a simple process.
Q15. How can we convert a saturated solution into an unsaturated one by heating?
A saturated solution contains the maximum amount of solute that can dissolve at a specific temperature. When heated:- The solvent molecules gain kinetic energy.
- They vibrate and move apart, creating more space.
- This allows additional solute particles to dissolve.
- As a result, the solution becomes unsaturated.
Thus, heating a saturated solution enables it to accommodate more solute, transforming it into an unsaturated solution.
Q16. What is the difference between fog and smoke?
Fog is a colloidal solution consisting of tiny liquid droplets suspended in gas. In contrast, smoke is a colloidal solution made up of tiny solid particles dispersed in gas.
- Fog: Liquid droplets in gas.
- Smoke: Solid particles in gas.
Q17: If 20g of salt is present in 220 g of solution, calculate the concentration of the solution.
Concentration of solution = Mass of solute/(Mass of solute + Mass of solvent) × 100
Mass Solute = 20g
Mass of solute + solvent = 220g
∴ Concentration of solution = 20/220 × 100 = 9.09%
Long Answer Type Questions
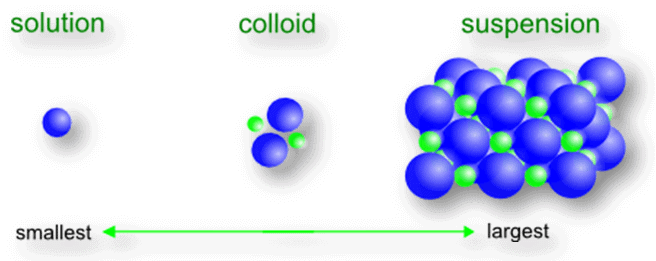
Q1. Give the difference between a true solution, a colloidal solution and a suspension.
The difference between true solution, colloidal solution and suspension
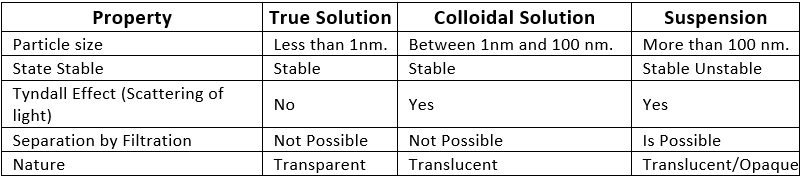
Q2. State the different types of colloids with examples.
Q3. (a) Define solution.
(b) Give different types of solutions with one example each.
(a) Solution: It is a homogeneous mixture of two or more substances. It consists of solute and solvent.
(b) Different types of solution:
- Based on solvent—Aqueous and non-aqueous Aqueous solution has water as solvent (sugar + water) Non-aqueous solution has some other solvent but not water.
Example: (sulphur + carbon disulphide) - Depending on the amount of solute dissolved in solvent—Dilute solution and concentrated solution.
- Dilute solution—Less amount of solute particles are present in a solvent.
Concentrated solution—Amount of solute present in its maximum capacity in a solvent. - Amount of solute present in its maximum capacity at a given temperature—Saturated and unsaturated solution.
- Saturated Solution—It is a solution in which no more solute can further dissolve in a given solvent at a given temperature.
- Unsaturated Solution—It is a solution in which some more solute can dissolve in a solvent at a given temperature.
- Depending on the size of solute particles:
(i)True solution Size is very small and particles cannot be seen through naked eyes
(ii) Suspension Size is very big and can be seen through naked eyes
(iii) Colloid Size is intermediate between true solution and suspension
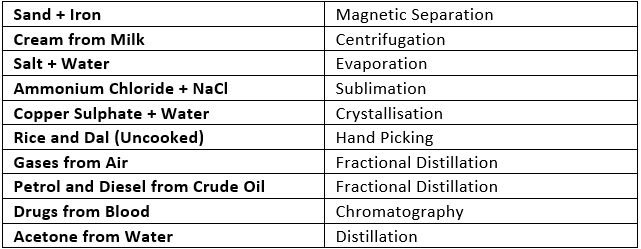
Q4. How can you separate the following mixtures?
(a) Sand + iron
(b)Cream from milk
(c) Salt + water
(d) Ammonium chloride + NaCl
(e) Copper sulphate + water
(f) Rice and dal (uncooked)
(g) Gases from air
(h) Petrol and diesel from crude oil
(i) Drugs from blood
(j) Acetone from water
Value-Based Questions
Q1. Anil’s sister accidentally added some water to the bottle containing olive oil, and she was afraid of the scolded. Anil helped his sister and separated the water from the olive oil using a bottle as a separating funnel.
(a) What is the principle of using and working of a separating funnel?
(b) Suggest two separation techniques used to separate liquid mixtures.
(c) What value of Anil is seen in the above case?
(a) The principle of the separating funnel is the difference in the densities of two immiscible liquids.
(b) Liquid mixtures can be separated by distillation and fractional distillation.
(c) Anil showed the value of helping, caring and responsible behaviour.
Q2. Preeti saw a labourer entering the sewage manhole immediately after removing the lid. She promptly stopped the labour from entering the manhole and told him to wait for some time before he entered it.
(a) What will happen if the labourer immediately enters the manhole for cleaning after removing the lid?
(b) Name the main gases that are released from the manhole.
(c) What value of Preeti is seen in the above act?
(a) He could die from suffocation due to inhalation of poisonous gases released from sewage.
(b) Gases released from the sewage manhole are methane, carbon dioxide and hydrogen sulphide.
(c) Preeti showed moral responsibility and awareness as a citizen.
Q3. Prasanna wanted to buy a deodorant from the shop. While buying a bottle, he felt that it was slightly heavier than the usual deodorant bottle that he purchased every time. He read the weight mentioned on the bottle and told the shopkeeper to weigh the same. He found the bottle was heavy, and on opening the deodorant bottle, he found it half-filled with water. He complained about the matter to the consumer authority.
(a) Define density.
(b) Apart from water, what is the other substance that some shopkeepers add to the deodorant?
(c) What value of Prasanna is reflected in this act?
(a) Density of any substance is defined to be the mass of the substance per unit volume.
(b) One can add some cheap gases or compressed air in the deodorant bottles.
(c) Prasanna showed leadership, awareness of consumer rights, and responsibility.
Q4. Rita’s father always got his vehicle checked for pollution control. He got it tested for the aerosol if released by his car. He also uses unleaded petrol and makes use of public transport wherever possible. He sparingly uses his car.
(a) What is aerosol?
(b) What happens when smoke released from a vehicle mixes with fog?
(c) What values of Rita’s father are reflected here?
(a) An aerosol is a colloid in which solid or liquid particles are dispersed in a gas.
Example: Smoke.
(b) When smoke mixes with fog it forms smog.
(c) Rita’s father reflects environmental awareness, responsibility, and good citizenship.





























