Solutions of Carbon And Its Compounds (Page No - 262) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 1:
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas ?
Solution :
Carbon dioxide (CO2) gas is evolved in the reaction. When passed through lime water, it turns lime water milky.
Question 2:
Which of the following will give brisk effervescence with sodium hydrogencarbonate and why ? CH3COOH, CH3CH2OH
Solution :
CH3COOH will give brick effervescence. Being acid, it reacts with sodium hydrogencarbonate to produce carbon dioxide gas.
Question 3:
Name the functional group present in an organic compound which gives brisk effervescence with NaHC03.
Solution :
Carboxylic acid group, -COOH gives brisk effervescence with NaHCO3
Question 4:
Name the hydrocarbon formed when ethanol is heated with cone. H2S04 at 170°C ? What is this reaction known as ?
Solution :
Ethene is formed when ethanol is heated with conc. H2SO4 at 170oC. This reaction is called dehydration.
Question 5:
Why does ethyne (acetylene) burn with a sooty flame ?
Solution :
Ethyne (acetylene) burn with a sooty flame because ethyne is an unsaturated hydrocarbon and the percentage of carbon in these hydrocarbons is comparatively higher which does not get oxidised completely in oxygen of air.
Question 6:
Name the product formed when hydrogen is added to ethene.
Solution :
Ethane is formed when hydrogen is added to ethene.
Question 7:
Explain why, ethene decolourises bromine water whereas ethane does not.
Solution :
Ethene decolourises bromine water because ethene is an alkene. And all alkenes and alkynes are unsaturated compounds which decolourise bromine water. On the other hand, ethane being an alkane is a saturated compound which does not decolourise bromine water.
Question 8:
Name two catalysts which can be used in the hydrogenation of unsaturated compounds.
Solution :
Nickel or palladium can be used as catalyst in the hydrogenation of unsaturated compounds.
Question 9:
State two disadvantages of incomplete combustion.
Solution :
Disadvantages of incomplete combustion:
(i) It leads to the formation of soot which is nothing but unburnt carbon which pollutes the atmosphere, blackens cooking utensils.
(ii) It leads to the formation of an extremely poisonous gas called carbon monoxide.
Question 10:
What happens when (give chemical equation) :
Sodium reacts with ethanol (ethyl alcohol)
Solution :
When Sodium reacts with ethanol (ethyl alcohol), hydrogen gas is evolved.
2C2H5OH + 2Na → 2C2H5O-Na+ + H2
Question 11:
Describe one reaction of ethanol.
Solution :
Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas. This reaction is used as a test for ethanol. When a small piece of sodium metal is put into ethanol in a dry test tube, rapid effervescence is produced due to evolution of hydrogen gas.
2C2H5OH + 2Na → 2C2H5O-Na+ + H2
Question 12:
Name one liquid carbon compound which is being used as an additive in petrol in some countries.
Solution :
Ethanol is used as an additive in petrol.
Question 13:
What are the raw materials required for making soap in a laboratory (or at home) ?
Solution :
(i) Vegetable oil (like castor oil, cottonseed oil or soyabean oil)
(ii) Sodium hydroxide (caustic soda)
(iii) Sodium chloride (common salt)
Question 14:
Would you be able to check whether water is hard by using a detergent ? Why ?
Solution :
No, we would not be able to check the hardness of water by using a detergent because a detergent forms lather easily even with hard water.
Question 15:
Describe a test for carboxylic acids.
Solution :
Litmus test: Some blue litmus solution is added to the organic compound (to be tested). If the blue litmus solution turns red, it shows that the organic compound is acidic in nature and hence it is a carboxylic acid.
Question 16:
Why is the conversion of ethanol into ethanoic acid an oxidation reaction ?
Solution :
Oxidation means controlled combustion. When ethanol is heated with alkaline potassium permanganate solution (or acidified potassium dichromate solution), it gets oxidised to ethanoic acid. It is called an oxidation reaction because oxygen is added to it during this conversion.

Question 17:
Explain why, alkanes are excellent fuels.
Solution :
Alkanes burn in air to produce a lot of heat due to which they are known to be excellent fuels.
Question 18:
Name one chemical compound which can be used to distinguish between ethanol and ethanoic acid.
Solution :
Sodium hydrogencarbonate can be used to distinguish between ethanol and ethanoic acid.
Question 19:
Complete the following equations :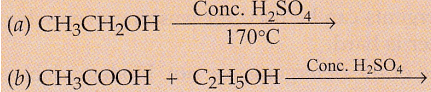
Solution :
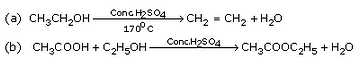
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Carbon And Its Compounds (Page No - 262) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What is the definition of carbon compounds? |  |
| 2. What are the different types of carbon compounds? |  |
| 3. How do carbon compounds form? |  |
| 4. What are some examples of carbon compounds? |  |
| 5. What are the applications of carbon compounds? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















