Solutions of Chemical Reactions and Equations (Page No - 46) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 16:
What type of chemical reactions take place when :
(a) a magnesium wire is burnt in air ?
(b) lime-stone is heated ?
(c) silver bromide is exposed to sunlight ?
(d) electricity is passed through water ?
(e) ammonia and hydrogen chloride are mixed ?
Solution :
(a) Combination.
(b) Decomposition.
(c) Decomposition.
(d) Decomposition.
(e) Combination.
Question 17:
What type of chemical reactions are represented by the following equations ?
- A + BC ——- > AC + B
- A + B ——- > C
- X ——- > Y + Z
- PQ + RS —— > PS + RQ
- A2O3 + 2B —–> B2O3 + 2A
Solution :
- Displacement reaction.
- Combination reaction.
- Decomposition reaction.
- Double displacement reaction.
- Displacement reaction.
Question 18:
Balance the following chemical equations :![]()
Solution :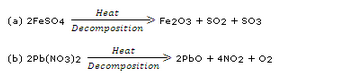
Question 19:
Which of the following is a combination and which is a displacement reaction ?
(a) Cl2 + 2KI ——-> 2KCl + I2
(b) 2K + Cl2 ——–> 2KCl
Solution :
(a) Displacement reaction.
(b) Combination reaction.
Question 20:
What type of reactions are represented by the following equations ?
(a) CaO + CO2 ———- > CaCO3
(b) 2Na + 2H2O —— > 2NaOH + H2
(c) Mg + CuS04——— > MgSO4 + Cu
(d) NH4NO2 ——- > N2 + 2H2O
(e) CuS04 + 2NaOH ——– > Cu(OH)2 + Na2SO4
Solution :
(a) Combination reaction.
(b) Displacement reaction.
(c) Displacement reaction.
(d) Decomposition reaction.
(e) Double displacement reaction.
Question 21:
In the following reaction between lead sulphide and hydrogen peroxide :
PbS (s) + 4H2O2 (aq) ————-> PbS04 (s) + 4H2O (l)
(a) Which substance is reduced ?
(b) Which substance is oxidised ?
Solution :
(a) H2O2
(b) PbS
Question 22:
Identify the component oxidised in the following reaction :
H2S + Cl2 ————->S + 2HCl
Solution :
H2S
Question 23:
When S02 gas is passed through saturated solution of H2S, the following reaction occurs :
SO2 + 2H2S ———– > 2H2O + 3S
In this reaction, which substance is oxidised and which one is reduced ?
Solution :
Substance oxidised: H2S
Substance reduced: SO2
Question 24:
Fill in the following blanks with suitable words :
(a) The addition of oxygen to a substance is called ……………………… whereas removal of oxygen is
called……
(b) The addition of hydrogen to a substance is called……… whereas removal of hydrogen is called………..
(c) Anti-oxidants are often added to fat containing foods to prevent………….. due to oxidation.
Solution :
(a) Oxidation; reduction.
(b) Reduction; oxidation.
(c) Rancidity.
Question 25:
What is an oxidation reaction ? Identify in the following reaction
(i) the substance oxidised, and (ii) the substance reduced :
ZnO + C ———— > Zn + CO
Solution :
Oxidation Reaction: The addition of oxygen (or removal of hydrogen) to a substance is called oxidation.
(i) C (ii) ZnO
Question 26:
(a) What is a redox reaction ? Explain with an example.
(b) When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced ? Why ?
(c) In the reaction represented by the equation :
MnO2 + 4Hl ———–> MnCl2 + 2H2O + Cl2
- name the substance oxidised.
- name the oxidising agent.
- name the substance reduced.
- name the reducing agent.
Solution :
(a) The oxidation and reduction reactions occurring together are called a redox reaction. Example:
In this reaction, copper oxide is being reduced to copper whereas hydrogen is being oxidised to water.
(b) Magnesium is oxidised as addition of oxygen to magnesium takes place leading to formation of magnesium oxide.
![]()
(c)
- HCl
- MnO2
- MnO2
- HCl
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Chemical Reactions and Equations (Page No - 46) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What is a chemical reaction? |  |
| 2. How are chemical reactions represented? |  |
| 3. What are the different types of chemical reactions? |  |
| 4. How can chemical reactions be balanced? |  |
| 5. Why are chemical equations important in chemistry? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















