Class 9 Exam > Class 9 Notes > Science Class 9 > Structure of Atom and Various Models of Atom
Structure of Atom and Various Models of Atom | Science Class 9 PDF Download
| Table of contents |

|
| Dalton's Atomic Theory |

|
| Thomson's Model of an Atom |

|
| Rutherford's Model of an Atom |

|
| Bohr's Model of Atom |

|
| Neutrons |

|
| Atomic Structure |

|
Dalton's Atomic Theory
Major postulates of Dalton's Atomic Theory are:
- According to John Dalton, all matter was composed of a small particle called an atom.
- Atom is a Greek word and its meaning is indivisible i.e. an ultimate particle which cannot be further subdivided.
- Atom is the smallest indivisible part of matter which takes part in the chemical reaction.
- Atoms are neither created nor destroyed.
- Atoms of the same element are similar in size, mass and characteristics however, atoms of different elements have different sizes, masses and characteristics.
 Dalton's ideas proved foundational to modern atomic theory. However, one of his underlying assumptions was later shown to be incorrect. Dalton thought that atoms were the smallest units of matter- tiny, hard spheres that could not be broken down any further. This assumption persisted until experiments in physics showed that the atom was composed of even smaller particles.
Dalton's ideas proved foundational to modern atomic theory. However, one of his underlying assumptions was later shown to be incorrect. Dalton thought that atoms were the smallest units of matter- tiny, hard spheres that could not be broken down any further. This assumption persisted until experiments in physics showed that the atom was composed of even smaller particles.
Question for Structure of Atom and Various Models of Atom
Try yourself:According to Dalton's Atomic Theory, all matter consists of indivisible particles called ?
View Solution
Cathode Ray Experiment
- J. J. Thomson discovered the existence of electrons.
- He did this using a cathode ray tube, which is a vacuum-sealed tube with a cathode and anode on one end that created a beam of electrons travelling towards the other end of the tube.
- The air inside the chamber is subjected to high voltage and electricity flows through the air from the negative electrode to the positive electrode.
- The characteristics of cathode rays (electrons) do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
- The experiment showed that the atom was not a simple, indivisible particle and contained at least one subatomic particle – the electron.
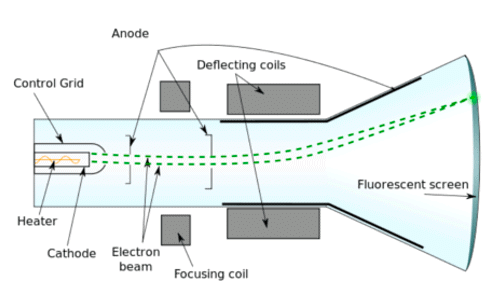 Apparatus of the Experiment
Apparatus of the Experiment
Electrons
- Electrons are the negatively charged sub-atomic particles of an atom.
- The mass of an electron is considered to be negligible, and its charge is -1.
- The symbol for an electron is e–.
- Electrons are extremely small.
- They are found outside the nucleus.
Thomson's Model of an Atom
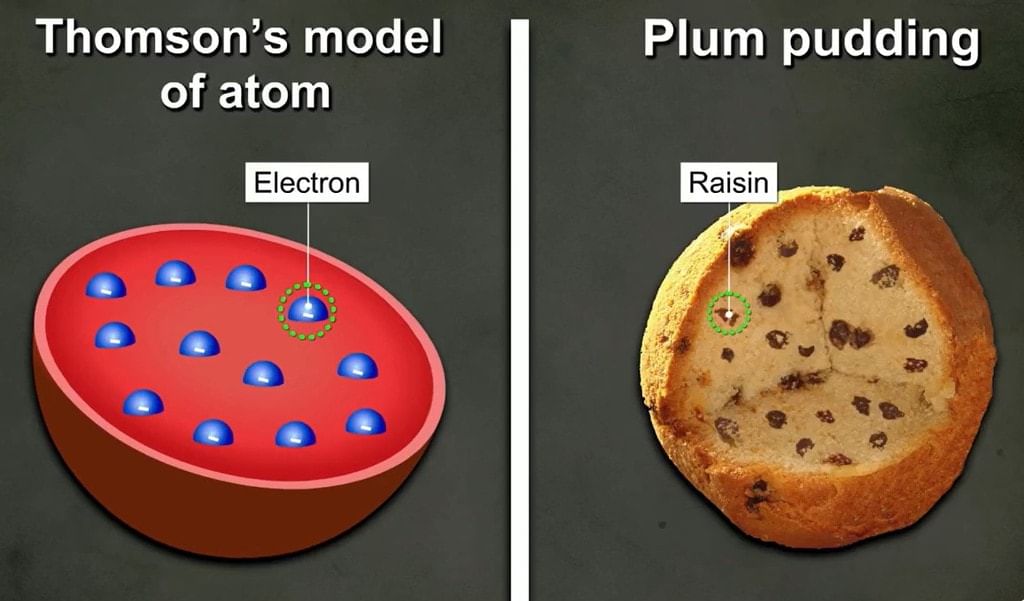
- A detailed model of the atom was first proposed by Sir J.J. Thomson.
- Thomson proposed that an atom consists of a uniform sphere of positive charge in which the electrons are distributed more or less uniformly.
- Thomson proposed the model of an atom similar to that of a Christmas pudding.
- The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding.
- We can also think of a watermelon, the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electron is sudden in the positively charged sphere, like the seeds in the watermelon.
Drawbacks of Thomson's Model of an Atom
- An important drawback of this model is that the mass of the atoms is considered to be evenly spread over that atom.
- It is a static model. It does not explain the movement of electrons.
- It could not explain the stability of an atom.
- The plum pudding atomic model lacked experimental evidence and hence Rutherford conducted many experiments in order to determine the structure of an atom.
Rutherford's Model of an Atom
- In 1911, scientist "Ernest Rutherford" gave a new picture of the structure of atoms by his α-particle scattering experiment & proposed the structure of atoms. α particles are charged particles having 2 units of positive charge and 4 units of mass, that is α-particles (2He4) are doubly charged helium atoms (He+2).
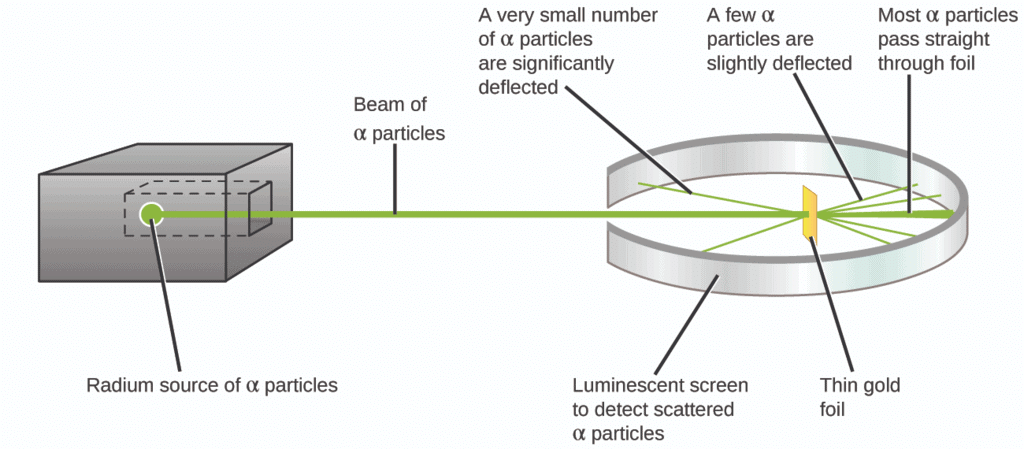
- When fast-moving alpha particles strike very thin gold foil in a vacuum, it was found that:
(i) Most of the fast-moving α-particles passed straight through the gold foil, without any deflection from their original part.
(ii) Some of the α-particles were deflected by the foil at small angles and few are deflected through large angles.
(iii) Very few alpha particles completely rebound on hitting the gold foil and turn back on their path.
Question for Structure of Atom and Various Models of Atom
Try yourself:An alpha particle is also known as:
View Solution
Conclusion of Rutherford Experiment
- Rutherford concluded from the α-particle scattering experiment that most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space. A very small fraction of α-particles were deflected by 180°, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
- On the basis of this experiment, Rutherford put forward the nuclear model of an atom, which has the following features:
(i) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of the atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in a well-defined orbit.
(iii) The size of the nucleus is very small compared to the size of the atom.
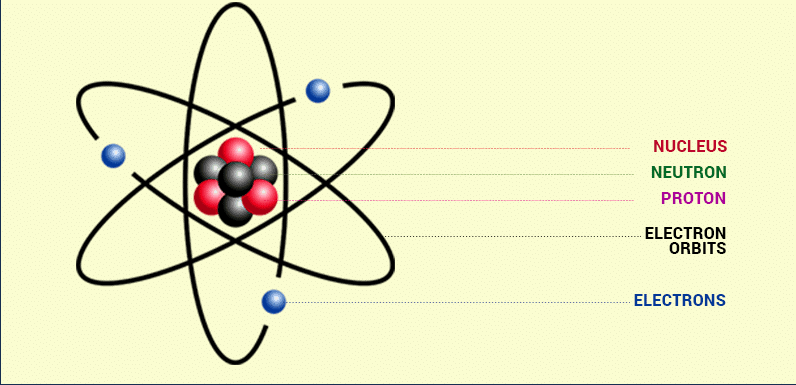 Rutherford's Model of Atom
Rutherford's Model of Atom
Drawbacks of Rutherford's Model of an Atom
- According to Maxwell, if an electrically charged particle revolves around a circular path, then it always radiates out energy.
- Thus, if an electron moves around the nucleus, it must continuously radiate out energy and hence, gradually move towards the nucleus in a spiral path, till it collides with the nucleus.
- One of the drawbacks of the Rutherford model was also that he did not say anything about the arrangement of electrons in an atom which made his theory incomplete.
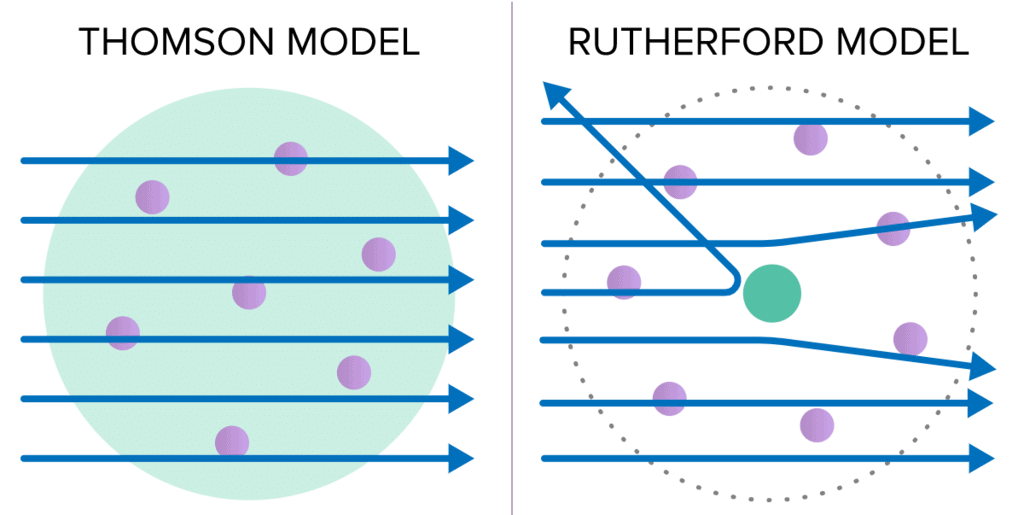 Different observations on the basis of the Thomson Model & Rutherford Model
Different observations on the basis of the Thomson Model & Rutherford Model
Bohr's Model of Atom
- Rutherford's Model of the atom was unable to explain certain observations with regard to the atom that is the stability of the atom and the occurrence of the atomic spectra.
- Niels Bohr accepted Rutherford's idea that the positive charge and most of the mass of an atom is concentrated in its nucleus with the electrons present at some distance away.
- It is a quantum mechanical model. This model was based on the quantum theory of radiation or plank theory and the classical law of physics.
According to Bohr's Theory
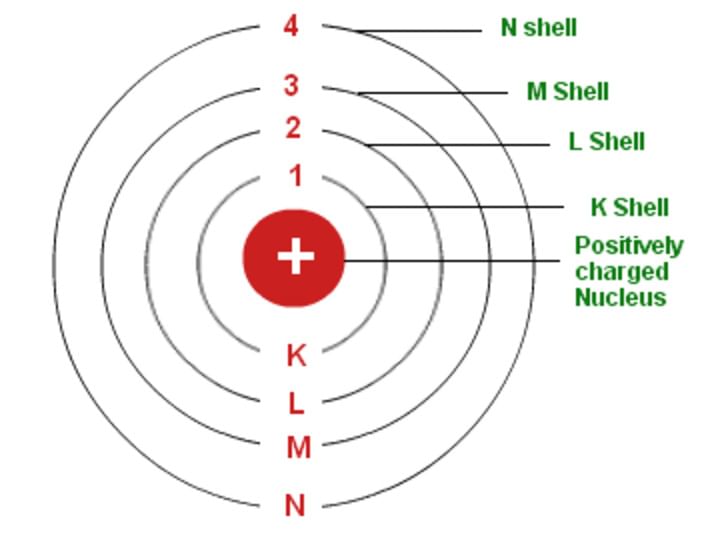
- Electrons revolve around the nucleus in well-defined orbits or shells each shell having a definite amount of energy associated with the electrons in it. Therefore these shells are also called energy levels.
- The energy associated with the electrons in an orbit increases as the radius of the orbit increases. These shells are known as K, L, M, N..... starting from the one closest to the nucleus.
- An electron in a shell can move to a higher or lower energy shell by absorbing or releasing a fixed amount of energy.
- The amount of energy absorbed or emitted is given by different energies associated with the two levels. Thus
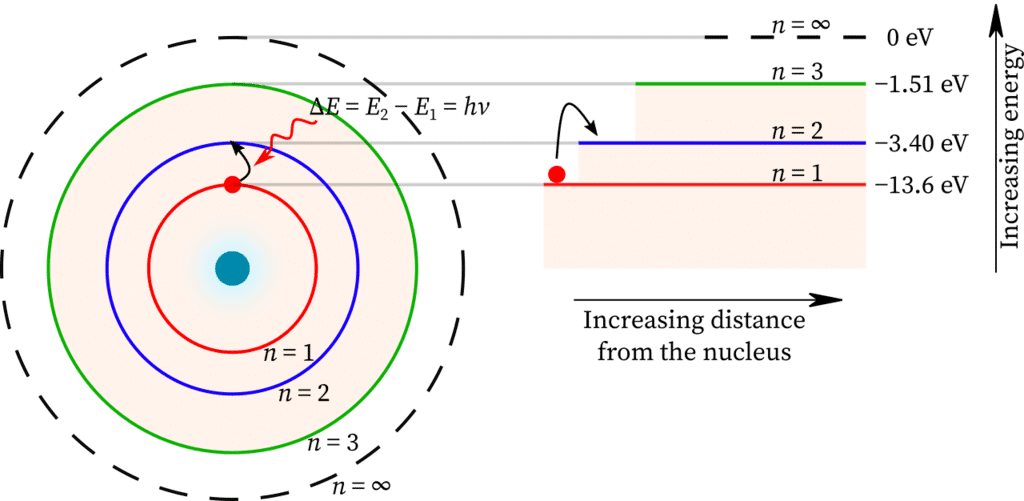
- Energy absorbed ΔE = E2 – E1 = hν. The energy emitted ΔE = E2 – E1 = hv, where h is Planck's constant and ν is the frequency of the radiation.
Drawbacks of Bohr’s Model of an Atom
- Bohr’s model of an atom failed to explain the Zeeman Effect (effect of magnetic field on the spectra of atoms).
- It also failed to explain the Stark effect (effect of electric field on the spectra of atoms).
- It violates the Heisenberg Uncertainty Principle.
- It could not explain the spectra obtained from larger atoms.
Question for Structure of Atom and Various Models of Atom
Try yourself:Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(ii) Bohr’s atomic model
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(ii) Bohr’s atomic model
View Solution
 |
Download the notes
Structure of Atom and Various Models of Atom
|
Download as PDF |
Download as PDF
Neutrons
Discovery of Neutrons- In 1932, "Chadwick" bombarded beryllium with a stream of α-particles. He observed that penetrating radiation was produced which was not affected by electric & magnetic fields.
- This radiation consists of neutral particles, which are called neutrons. The nuclear reaction can be shown as:
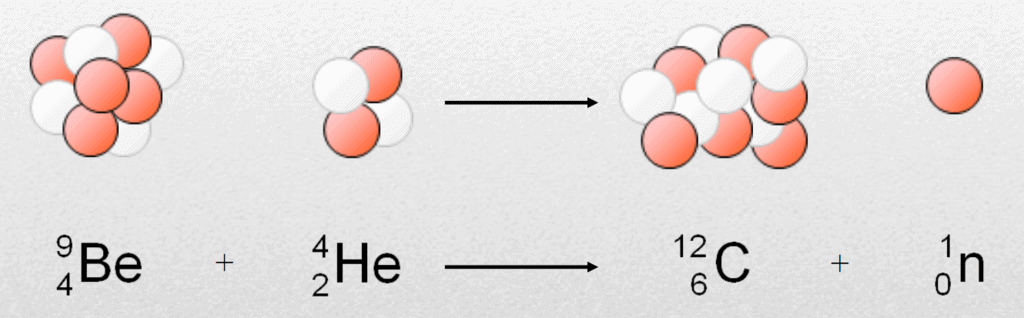
- The neutron is a fundamental constituent of atoms and is located in the nucleus.
Characteristics of a Neutron
- Mass: The relative mass of a neutron is almost equal to that of the proton. In fact, the relative mass of a neutron is 1.0087 (1.008) amu and that of the proton is 1.0073 (1.008) amu.
- Charge: Neutrons are electrically neutral and have no electric charge. With the discovery of neutrons, we can explain why the atomic mass of helium is 4 amu.
- Atomic mass = Mass of proton + Mass of neutron.
Atomic Structure
- An atom is made up of three subatomic particles: electron, proton & neutrons. These three particles are called fundamental particles of matter.
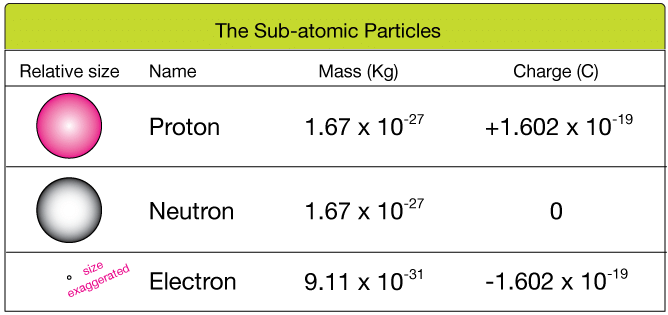
- There are two parts of an atom:
(i) Nucleus
(ii) Outer part - Nucleus: The size of the nucleus of an atom is very small in which neutrons and protons are present, so almost the entire mass of the atom is situated in the nucleus. Protons & neutrons present in the nucleus are collectively termed as nucleons. The size of the nucleus in the atom is 10-15m.
Mass number (A) = Number of protons in the nucleus (p) + Number of Neutrons (n) - Outer Part: In the outer part, electrons move around the nucleus in fixed orbits. These orbits are called energy levels.
The document Structure of Atom and Various Models of Atom | Science Class 9 is a part of the Class 9 Course Science Class 9.
All you need of Class 9 at this link: Class 9
|
84 videos|384 docs|61 tests
|
FAQs on Structure of Atom and Various Models of Atom - Science Class 9
| 1. What is Dalton's Atomic Theory? |  |
| 2. How did Thomson contribute to our understanding of the atom? |  |
Ans. Thomson's Model of an Atom, proposed by J.J. Thomson in 1897, introduced the concept of the electron. He theorized that atoms were made up of a positively charged "pudding" with negatively charged electrons scattered throughout. This model provided the first evidence of subatomic particles and challenged the idea of atoms being indivisible.
| 3. What is Rutherford's Model of an Atom? |  |
Ans. Rutherford's Model of an Atom, proposed by Ernest Rutherford in 1911, suggested that atoms have a small, dense, positively charged nucleus at the center, surrounded by negatively charged electrons in orbit. This model was based on Rutherford's famous gold foil experiment, which showed that most of the atom's mass and positive charge is concentrated in the nucleus.
| 4. How did Bohr's Model contribute to the understanding of atomic structure? |  |
Ans. Bohr's Model of Atom, proposed by Niels Bohr in 1913, built upon Rutherford's model by introducing the concept of energy levels or electron shells. Bohr proposed that electrons occupy specific energy levels around the nucleus and can jump between these levels by gaining or losing energy. This model helped explain the stability of atoms and the emission and absorption of light by atoms.
| 5. What is the role of neutrons in the atomic structure? |  |
Ans. Neutrons are subatomic particles with no electrical charge that are found in the nucleus of an atom. They contribute to the mass of the atom but do not affect its overall charge. Neutrons help stabilize the nucleus by balancing the repulsive forces between positively charged protons. The number of neutrons in an atom can vary, resulting in different isotopes of an element.
Related Searches























