Synthesis, Reactivity and Properties of Pyrrole | Organic Chemistry PDF Download
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH.It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3.
Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls.
Synthesis of Pyrrole
Pyrrole is prepared industrially by treatment of furan with ammonia in the presence of solid acid catalysts, like SiO2 and Al2O3.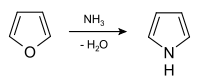
Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine. Several methods of synthesis of the pyrrole ring have been described.
Hantzsch pyrrole synthesis
Main article: Hantzsch pyrrole synthesis The Hantzsch pyrrole synthesis is the reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles.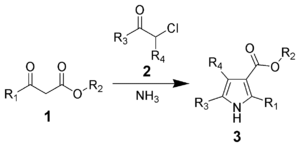
Knorr pyrrole synthesis
The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound.The method involves the reaction of an α-aminoketone (1) and a compound containing a methylene group α to (bonded to the next carbon to) a carbonyl group (2).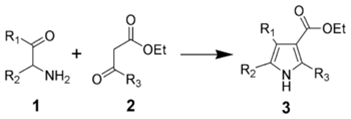
Paal–Knorr pyrrole synthesis
Main article: Paal–Knorr pyrrole synthesis In the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.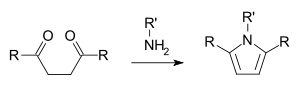
Reactivity of Pyrrole
Due to its aromatic character, pyrrole is difficult to hydrogenate, does not easily react as a diene in Diels–Alder reactions, and does not undergo usual olefin reactions. Its reactivity is similar to that of benzene and aniline, in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles polymerize easily, and thus many electrophilic reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including protected pyrroles) have been used in a broad range of transformations.
Reaction of pyrrole with electrophiles
Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate.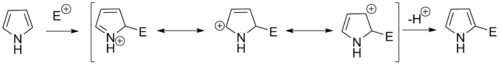
Pyrroles react easily with nitrating (e.g. HNO3/Ac2O), sulfonating (Py·SO3), and halogenating (e.g. NCS, NBS, Br2, SO2Cl2, and KI/H2O2) agents. Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.
Acylation
Acylation generally occurs at the 2-position, through the use of various methods. Acylation with acid anhydrides and acid chlorides can occur without a catalyst; alternatively, a Lewis acid may be used. 2-Acylpyrroles are also obtained from reaction with nitriles, by the Houben–Hoesch reaction. Pyrrole aldehydes can be formed by a Vilsmeier–Haack reaction. N-Acylation of simple pyrrole does not occur.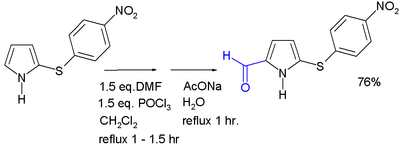
Alkylation
Electrophilic alkylation of simple pyrrole is uncommon. Alkylation to form enones at C2 has been seen.
Reaction of deprotonated pyrrole
The NH proton in pyrroles is moderately acidic with a pKa of 16.5. Pyrrole can be deprotonated with strong bases such as butyllithium and sodium hydride. The resulting alkali pyrrolide is nucleophilic. Treating this conjugate base with an electrophile such as iodomethane gives N-methylpyrrole. N-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with Li, Na, and K) and more solvating solvents lead to N-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom. In the cases of N-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.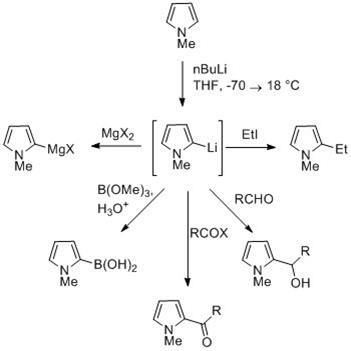
Substitution at C3 can be achieved through the use of N-substituted 3-bromopyrrole, which can be synthesized by bromination of N-silylpyrrole with NBS.
Reductions
Pyrroles can undergo reductions to pyrrolidines and to pyrrolines. For example, Birch reduction of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
Cyclization reactions
Pyrroles with N-substitution can undergo cycloaddition reactions such as [4+2]-, [2+2]-, and [2+1]-cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.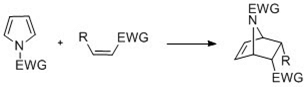
Pyrroles can react with carbenes, such as dichlorocarbene, in a [2+1]-cycloaddition. With dichlorocarbene, a dichlorocyclopropane intermediate is formed, which breaks down to form 3-chloropyridine (the Ciamician–Dennstedt rearrangement).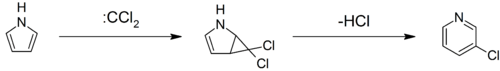
Pyrrole Properties
Physical properties.
Pyrrole is a colorless liquid, b.p. 131°C, which rapidly turns brown on exposure to air. Its odor is like that of chloroform. It is sparingly soluble in water but readily soluble in ethanol and ether.
Chemical Properties.
Chemically pyrrole shows the reactions of aromatic compounds. It is less aromatic than thiophene but more aromatic than furan. Some important reactions of pyrrole are:
Basic Character.
Pyrrole is a weak base (pKa = 3.4). It reacts with dilute hydrochloric acid to give crystalline hydrochloride. This salt is stable in the absence of oxygen; otherwise, polymerization rapidly occurs to produce a brown resin. The reason for the weak basic character of pyrrole is that the lone pair of electrons on nitrogen is involved in the formation of the delocalized π molecular orbital and is not available for the formation of a new bond with a proton. Furthermore, if a proton is added to the nitrogen atom by reaction with an acid, the resulting structure ceases to be aromatic and the resonance energy is lost. This makes the pyrrole cation very unstable in comparison to the free pyrrole and indicates why pyrrole is a weak base. Also, the Pyrrole cation behaves as a typically conjugated diene and undergoes polymerization readily.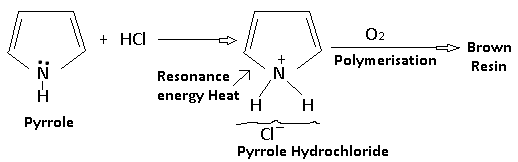
Acidic Character.
Pyrrole is not only a weak base but also a very weak acid (pKa =15), and forms a salt with potassium hydroxide; the imino hydrogen ins replaced by potassium.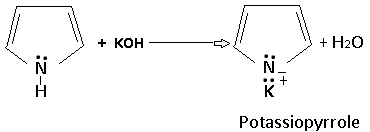
The reason for the acidic character of pyrrole is that the electron pair delocalization from nitrogen makes it positively charged and the increases the possibility of proton abstraction giving pyrrole anion. Furthermore, the pyrrole anion is stabilized by delocalization of the negative charge over the ring, and pyrrole anion has greater stability than pyrrole itself because, unlike pyrrole, there is no charge separation in the pyrrole anion, as is apparent from the following resonance structures: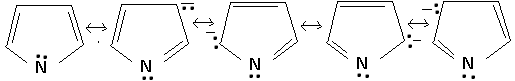
The acidic character of pyrrole is also evident from its reaction with methyl magnesium bromide to form a salt-like Grignard reagent.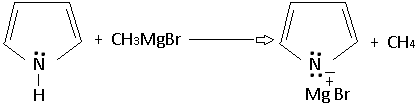
|
35 videos|92 docs|46 tests
|
FAQs on Synthesis, Reactivity and Properties of Pyrrole - Organic Chemistry
| 1. What is the synthesis of pyrrole? |  |
| 2. How reactive is pyrrole? |  |
| 3. What are the properties of pyrrole? |  |
| 4. How can pyrrole be used in organic synthesis? |  |
| 5. What are the biological activities of pyrrole compounds? |  |






















