The D-block elements - IIT JAM PDF Download
Ref: https://edurev.in/question/854971/Needed-a-Document-for-d-block-Related-P-Block-Elements-Part-4-Inorganic-Chemistry-IIT-JAM
The D-block elements find their applications at almost every scale of world. This article discusses all you need to know about them. But before getting into the D-block elements, let’s get a little idea of the modern periodic table first.
Modern Periodic Table
The modern periodic table is such well classified and arranged that one can investigate properties of elements in a variety of ways – as periods, groups or as blocks. The table, based on the modern periodic law – properties of elements are a periodic function of their atomic numbers – may not be a perfect classification, yet it can contribute explanations to most of the trends shown by elements in satisfactorily.
The most general classification in the table might be the 4 different blocks of elements – s, p, d and f blocks. While the s-block and p-blocks are relatively simpler to study due to the lesser numbers and simple underlying theories, any study of the f-block is constrained by their abnormal behaviour and complexity. This makes d-block elements the most interesting to learn due to the extent of their applications and the concepts explaining their properties.
The D-block elements
D-block elements occupy the space between the s-block and p-block in the periodic table. Since they bridge the two blocks and show a transition in the properties from the metals to the non-metals, they are also called Transition elements.
The valence electrons fills up the penultimate d-orbital energy level – according to the Aufbau principle, giving rise to four series of d-block elements – 3d, 4d, 5d and the 6d series. However 6d series is considered incomplete. In addition, as the criteria goes, the d-block elements Zinc, Cadmium and Mercury are not considered transition elements as they have completely filled d-orbitals in their ground and excited states.
D-block elements – Electronic configuration
D-block elements follow the general outer electronic configuration (n-1) d1-10 ns1-2. This however include many cases of deviation from the general electronic configuration. The exceptional cases arise mainly due:
1)Energy difference between the (n-1) d and ns orbitals
2)The relative stability of half-filled and completely filled d-orbits over other configurations.
This is illustrated in the example where Chromium (Z=24) which has a configuration 3d5 4s1 , having a half-filled d-orbital, against the expected configuration 3d4 4s2. The stability of completely filled d-orbital s evident in the configuration for Copper (Z=29), having configuration 3d10 4s1, not the expected configuration 3d9 4s2. Elements like Platinum (Pt), Palladium (Pd), Silver (Ag), Gold(Au) and most of the 4d series elements too exhibit exceptional electronic configuration.
Properties
D-block elements show typical metallic behaviour. These are characterised by high tensile strength, malleability, ductility, electrical and thermal conductivity as well as metallic lustre. Except Zinc (Zn), Cadmium (Cd), Mercury (Hg) and Manganese (Mn), they have one or more typical metallic lattices. Due to the larger extend of metallic bonding by virtue of d-electrons, they are hard and have high melting and boiling points. The group-12 elements (Zn, Cd and Hg) shows exception in this regard also.
Melting and Boiling point
The high melting and boiling points of d-block elements can be attributed to the involvement of d-orbital electrons in addition to the s-electrons in metallic bonding. Melting and boiling points increases as the d-orbital gets filled. This trend goes till d5 configuration and then decreases regularly as the orbital gets further filled and the electrons gets paired in the orbital. A point to be noted here is that Manganese (Mn) and Technetium (Tc) have abnormally low melting and boiling points.
- Exceptional case of Mercury – the liquid metal: Mercury is one among the two elements (other being Bromine(Br) ) and the only metal that exist in its liquid state at room temperature. This is unexpected for the general outlook of a metal and can be explained by the fact that 6s valence electrons of Mercury are more closely pulled by the nucleus, rendering those outer s-electrons less involved in metallic bonding. Still, Mercury shows good electrical conductivity.
Atomic and Ionic sizes
The ionic sizes shows gradual decrease as we move right across a series. This is because as we number of electrons increase, the nuclear charge too increase. Due to poor shielding of nuclear charge by d-electrons, the net increase in nuclear charge outweighs the effect of added electron, thereby reducing the size.
Similar trend and reasons can be observed for case of atomic radii as well, though the decrease is much gradual. In case of going down the series, the atomic radii shows increase. There is a huge jump from 3d to 4d in terms of atomic size, but the 4d and 5d series have a small difference only. This is explained on the basis of Lanthanoid contraction. In case of 5d series, the inner 4f orbitals are filled before 5d orbitals. The poor shielding ability of 4f electrons renders the outer electrons greater nuclear pull, causing lanthanoid contraction. Due to this, the expected increase in atomic size is compensated by increased nuclear pull, keeping the size nearly same.
Ionisation enthalpy
The same reasons that govern the trend in atomic size can explain the gradual increase in ionisation enthalphy as we move from left to right in a series. However, there exist large anomalies in this general trend. The anomalies arise due to the fact that removal of an electron alters the combined energy considerations of s and d orbital system. The concepts like hybridisation, pairing and exchange energy plays its role here, but let keep away such complexities for the time being. In simple terms, we can assume that the ionisation enthalpy will be high if removal of electron leads to deviation from a stable configuration – half or completely filled d-orbitals or hybridised orbitals.
Multiple oxidation states and stability
One of the most notable features of d-block elements are the variety of oxidation states exhibited by them. This leads to large number of compounds and pool of reactions involved. The number of oxidation states shown increases with the number of electrons available to lose/share. It decreases as number of paired electrons increase making the number of orbitals available for sharing less. Hence, the first and last elements shown less oxidation states and the elements with maximum number of oxidation states are towards the centre of the table. For example, Manganese (Z=25) shows states from Mn2+ to Mn7+, whereas Sc3+and Zn2+ are the only other states shown by Scandium (Z=23) and Zinc (Z=30) respectively.
In p-block, the heavier elements prefer lower oxidation states due to what is called inert pair effect. But in case of d-block elements, the higher oxidation states are more stable for heavier members in a group. For example, in group-6 ( Chromium (Cr), Molybdenum(Mo) and Tungsten(W) ), the +6 states for W and Mo are stable whereas Cr6+, as in potassium dichromate, easily reduces thus being a common oxidising agent.
Lower oxidation states in these metals are stabilised by ligands like CO, which are pi-electron donors, whereas the higher oxidation states are stabilised by electronegative elements like Fluorine(F) and Oxygen(O).Hence the high oxidation compounds of these metals are mainly fluorides and oxides.
Standard electrode potential
There are mainly the M2+/M and M3+/M2+ reduction potentials that are generally considered, for the states M2+ and M3+ respectively. The reduction potential values are governed by the ionisation enthalpies, size, electronic configuration of the ions and the energy levels of hybridised t2g and eg orbitals, which are beyond the scope of this article.
Magnetic properties
Paramagnetism, Diamagnetism and ferromagnetism are the general properties exhibited by substances. Here ferromagnetism is an extreme level of paramagnetism.
Parametric behaviour of d-block elements are due to presence of unpaired electrons. Such electrons contribute to ‘orbital magnetic moment’ and ‘spin magnetic moment’. However, for 3d series, orbital angular moment is negligible and the approximate spin-only magnetic moment is given by the formula:
μ = √n(n+2)
where n is the number of unpaired electrons. Its unit is Bohr Magneton (BM). For higher d-series, the actual magnetic moment includes components from orbital moment in addition to the spin moment.
Formation of coloured ions
The d-block element ions are known for the variety of colours their ions exhibit. The figure shown below are the coloured ions of few 3d series elements in aqueous medium, where H2O molecules are the ligands.
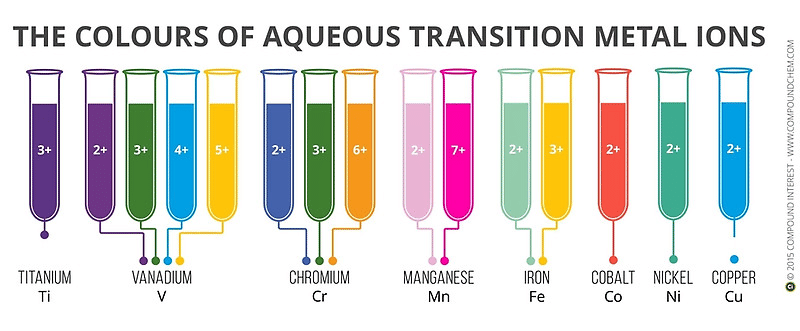
The colour shown is due to valence electron excitation and de-excitation. From the incident light, those frequencies are absorbed by the electrons whose energies equal the energy for excitation of the electron from the lower energy state of the d-orbital to the higher energy state of d-orbital. When this frequency of light is absorbed, the light transmitted through the salt solution exhibit the colour which is complementary to the frequency absorbed.
The colour of the ions varies with its oxidation state. The Cr6+ as in potassium dichromate yellow in colour, whereas Cr3+ and Cr2+ are generally green and blue respectively. This change in colour with respect to oxidation state can help us identify the end point in redox reactions, where the colour of the solution change as the oxidation state of reactants change.
One must also be aware that the colour of the compound is dependent on the complexing or co-ordianting group with the ions. The colour shown in aqueous medium (where the co-ordinating group is water molecule) may not be that exhibited by them with another complexing group. For example, Cu2+ shows light blue colour in presence of water as ligand. But in presence of ammonia as ligands, they show deep blue colour.
Formation of complexes
Co-ordination compounds are the compounds in which the central metal ion holds onto few electron rich neutral molecules or anions. D-orbitals form large number of co-ordination compounds. This is mainly due to three reasons:
1.Small size of the metal ion
2.High ionic charge
3.Presence of d-orbitals to make co-ordination bonds.
Catalytic properties
The d-block element ions in their various oxidation states are widely used in the industry as catalyst for quicker and efficient reactions to occur. Vanadium in its +5 state catalyses the Contact process. Haber’s process makes use of finely divided Iron and Nickel is used as catalyst in hydrogenation processes to optimise the reactions mentioned.
Formation of Interstitial compounds
To hear for the first time that VH0.6 and TiH1.7 are the chemical formulae for a compound was a confusing business. It was only later that those were representations of interstitial compounds. Interstitial compounds are formed when small atoms like Carbon, Hydrogen or Nitrogen occupy the voids between the atoms of the metal in its lattice.
Alloy formation
Alloys are homogeneous solid solutions of two metals or a metal with a non-metal. The elements in a particular row of d-block are nearly the same in size, which is a favourable condition for alloy formation.
Apart from the d-block, there are elements in f-block, whose valence electron goes to the f-orbital of preceding group. They are also called as the inner transition elements, and are placed away from the main table due to size considerations.
Knowledge of d-block elements is really significant, especially in industries due to their catalytic activity. The compounds like KMnO4 and K2Cr2O7 make use of the underlying principle of redox reaction and acts as redox indicators. The d-block metals are also extensively used for structural problems in engineering and architecture. Hence, developing a good understanding of the behaviour of these elements are surely interesting as well as informative.
FAQs on The D-block elements - IIT JAM
| 1. What are the D-block elements? |  |
| 2. How many D-block elements are there in the periodic table? |  |
| 3. What are the key properties of D-block elements? |  |
| 4. Why are D-block elements called transition metals? |  |
| 5. What are some applications of D-block elements? |  |















