Virtual Experiments - Acids, Bases and Salts - Class 10 PDF Download
Activity
Aim: To find ph of Salt.
Procedure
- Collect the following salt samples: Sodium chloride, potassium nitrate, aluminium chloride, zinc.
- Sulphate, copper sulphate, sodium acetate, sodium carbonate and sodium hydrogen carbonate.
- Check their solubility in water.
- Check the action of these solutions on litmus and find the pH using a pH paper.
► Which of the salts are acidic, basic or neutral? - Identify the acid or base used to form the salt.
Observations
- The pH value is greater than 7 at 25°C
Activity:
- Take a small amount of finely chopped onions along with some strips of clean cloth in a plastic bag.
- Tie up the bag tightly and leave it as such in a refrigerator for a night. In the morning, take two of these strips and check their odour.
- Now put a few drops of dilute HCl solution on one strip and a few drops of dilute NaOH solution on the other.
- Rinse both the cloth strips with water and again check their odour and note down in your notebook.
- You will see that onion will give different odour in HCl and NaOH.
- You can repeat the activity by taking dilute vanilla essence. Smell dilute vanilla essence.
- Now take some dilute HCl solution in one test tube and dilute NaOH solution in another test tube add a few drops of dilute vanilla essence to both the test tubes and shake well.
- Check the odour once again.
- You will feel different smells in both the test tubes.
- Lastly, you can repeat the activity by taking clove oil in place of vanilla essence.
- From this activity, we conclude that vanilla, onion or clove oil can also be used as olfactory indicators since these change their odour.
Activity
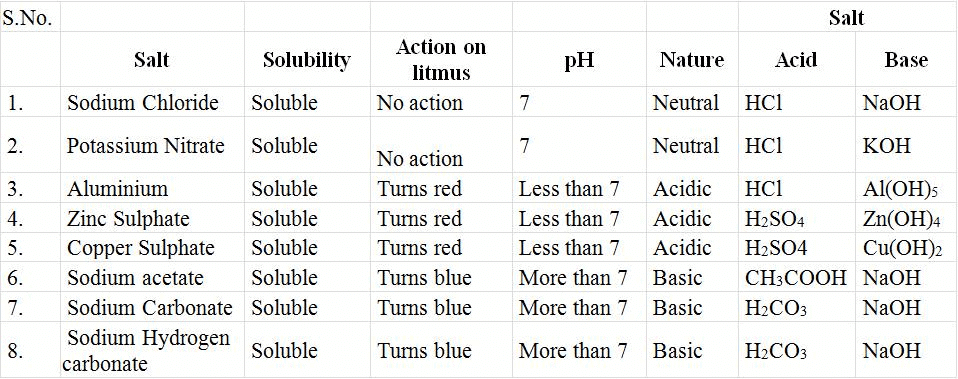
Materials required: Granulated zinc, Dilute sulphuric acid, Boiling tube, Matchbox.
Procedure
- Take about 5 ml of dilute sulphuric acid in a boiling tube.
- Add a few pieces of zinc metal into it and place an inverted boiling tube over its mouth.
- You can see the bubbles of hydrogen gas coming out of the mixture in the lower tube.
- After a few minutes, remove the upper boiling tube (Keeping its mouth downwards) near to its mouth. What do you see?
- The gas in the upper boiling tube burns with a blue flame producing the popping sound.
- Repeat a similar experiment with different acids and a few other metals. Write down your observations.
The reaction of Acids with Metals
Observation: Colourless, odourless gas is evolved. It burns explosively with a 'pop' sound.
Zn(s) + H2SO4 (dil) → ZnSO4 (aq) + H2(g)
Conclusion: Reactive metals react with dilute acid to liberate hydrogen gas. Metals which can displace hydrogen from dilute acid are known as active metals.
Example: Na, K, Zn, Fe, Ca, Mg etc.
Activity
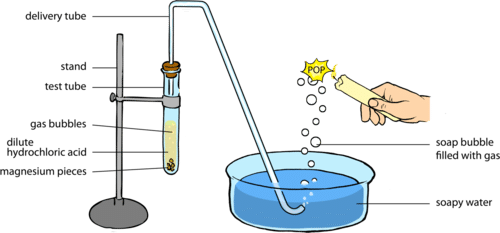
Aim: To study the reaction of sodium carbonate and sodium hydrogen carbonate with dilute acids.
Materials required: Sodium carbonate (Na2CO3), Sodium hydrogen carbonate, Hydrochloric acid (dil.), Limewater, Boiling tubes, Delivery tube.
Procedure
- Take about 0.5g of sodium carbonate in a boiling tube and 2-3 mL of freshly prepared limewater in another test tube.
- Set a delivery tube, as shown in fig. given alongside. Add about 2mL of dilute hydrochloric acid into the boiling tube containing sodium carbonate.
- A brisk effervescence is seen in the reaction mixture. Pass the gas evolved through limewater with the help of a delivery tube.
- What do you observe? The limewater turns milky.
- When an excess of carbon dioxide is passed, the milkiness disappears. Repeat the similar experiment with sodium hydrogen carbonate (NaHCO3), and if desired with other acids also.
The reaction of Acids with Sodium Carbonate and Sodium Hydrogen Carbonate
Conclusion: All acids decompose carbonates and hydrogen carbonates with the liberation of carbon dioxide gas.
Activity
Aim: To study the reaction of dilute acid with metal oxides (or basic oxides).
Materials required: Copper (II) oxide, Dilute hydrochloric acid, Test tube.
Procedure
- Take about 0.5g of copper (II) oxide (black in colour) in a test tube.
- Add dilute hydrochloric acid dropwise with occasional shaking till copper (II) oxide dissolve.
- Note the colour of the solution. I not it bluish-green? It is the solution of copper (II) chloride.
Conclusion: Acids react with metal oxides to give the corresponding salt & water.
Activity
Aim: To study a reaction of an acid, say hydrochloric acid with an alkali or base.
Materials required: The hydrochloric acid solution, sodium hydroxide solution, phenolphthalein indicator, Boiling tube, dropper, trough.
Procedure
- Take about 5 mL of a dilute solution of sodium hydroxide (NaOH) in a test tube. Add 2 drops of phenolphthalein indicator in it.
- The solution in the test tube turns pink.
- Now, add a dilute solution of hydrochloric acid (HCl) when the pink colour of the solution just disappears.
- Now, add a drop of sodium hydroxide solution and shake the test tube to mix the solution. What do you see? The solution turns pink.
- Add a drop of HCl solution to the solution in the test tube. The pink colour disappears.
- Keep repeating the addition of sodium hydroxide and hydrochloric acid solution one after the other and watch the appearance and disappearance of pink colour.
Conclusion
- This experiment shows that the addition of the HCl solution destroys the alkaline nature of NaOH.
- On the other hand, the addition of the NaOH solution destroys the acidic nature of HCl.
- That is, both NaOH and HCl appear to cancel the effect of each other.
- Such a reaction between an acid and alkali is called neutralisation.
Activity
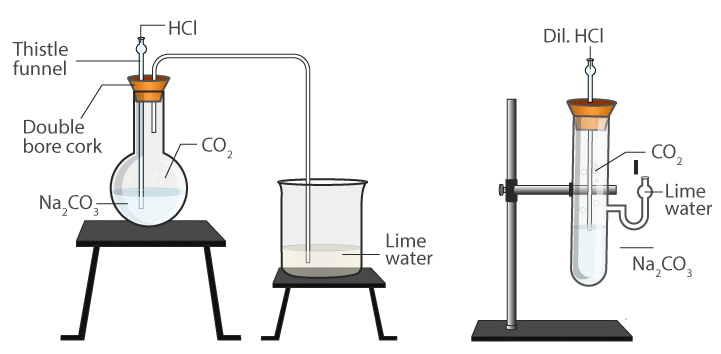
Aim: To find the characteristics common between acids and bases.
Materials required: Dilute hydrochloric acid, Dilute sulphuric acid, Dilute solution of sodium hydroxide, Ethanol, Glucose solution & Beaker, Carbon electrodes, Dry cells, bulb 1.5 V, Key.
Procedure
- Take a beaker and place two carbon electrodes into it.
- Connect the electrodes to a battery bulb through a key and a dry cell. Pour dilute hydrochloric acid into the beaker and press the key.
- Did the bulb glow? Perform a similar experiment with all the given solutions, and record your observations.
Acid Solution in Water Conducts Electricity
Observation
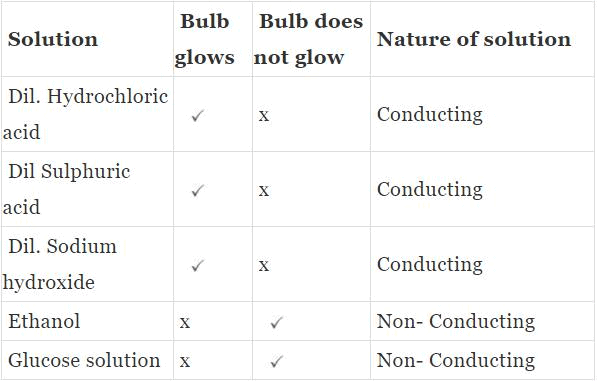
Conclusion: The solutions of acids and bases are good conductors of electricity. The solution of glucose and ethanol are nonconductors of electricity.
Activity
Aim: To show that acids furnish H+(aq) ions only in the presence of water.
Materials required: Common salt, Conc. sulphuric acid, anhydrous calcium chloride, blue litmus paper, boiling tube, delivery tube packed with anhydrous calcium chloride.
Procedure
- Take 0.5g of dry common salt in a dry boiling tube.
- Add a few drops of concentrated sulphuric acid over common salt in the boiling tube. What do you see?
- A colourless, irritating gas is evolved.
- Fit a cork carrying a calcium chloride packed delivery tube into the mouth of the boiling tube.
- Bring a dry blue litmus paper near the opening of the calcium chloride tube. Observe, if there is any change in colour. Colour of the litmus paper remains unchanged.
- Now, bring a moistened blue litmus paper near the mouth of the calcium chloride tube.
- Do you observe any change in the colour of litmus paper? Yes, blue litmus has changed to red.
Conclusion
- Dry HCl gas on coming in contact with dry blue litmus paper does not produce H+ ions, and hence the colour of the litmus paper does not change. So, we can say that separation of H+ ions from acid takes place only in the presence of water.
Activity
Aim: To show that water of crystallisation can be removed by heating.
Materials required: CuSO4.5H2O (Blue vitriol), boiling tube, burner, cork, delivery tube, test tube, clamp stand.
Procedure
- Take 2 g of CuSO4.5H2O in a boiling tube fitted in a clamp stand.
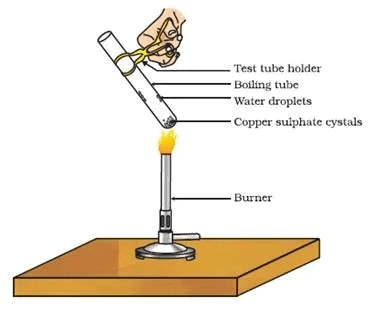
Removing Water of Crystallisation
- Observe its colour. Fit it with cork, and delivery tube bent at two right angles which dip into a test tube.
- Heat crystals in a boiling tube. Observe vapours being condensed in the test tube.
- Cool the crystals and add few drops of water into it
Observation
- Water vapours get condensed in a test tube, and the colour of blue crystals changes into white.
- On adding water to anhydrous copper sulphate, it changes into blue again.
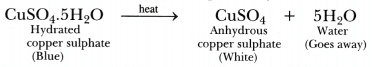
Conclusion: Crystalline substances have water of crystallization which is lost on heating.
FAQs on Virtual Experiments - Acids, Bases and Salts - Class 10
| 1. What are acids, bases, and salts? |  |
| 2. How can we identify whether a substance is an acid, base, or salt? |  |
| 3. What are the common uses of acids, bases, and salts? |  |
| 4. How do acids and bases react with each other? |  |
| 5. Can you give examples of common acids, bases, and salts? |  |

|
Explore Courses for Class 10 exam
|

|

















