Water: Structure, Properties & Hardness of Water | Chemistry for JEE Main & Advanced PDF Download
Water
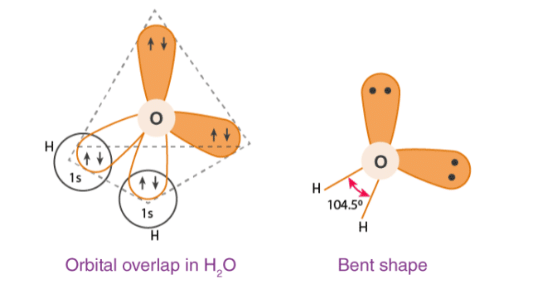
Water is the most abundant and widely distributed on the earth. It occurs in all the three physical states. H2O is a covalent molecule in which oxygen is sp3 hybridised. It has bent structure.
Physical Properties of Water
- Water is a colourless, odourless, tasteless liquid. It has abnormally high boiling point, freezing point, heat of vaporization due to hydrogen bonding.
- Pure water is not a good conductor so it is made conductor by adding small amount of acid or alkali.
- Density of ice (which is mass per unit volume) is lesser than that of water and it floats over water.
- Water has maximum density at 4°0.
- Water is a highly polar solvent with high dielectric constant 78.39. It interacts with polar or ionic substances effectively with the release of considerable amount of energy due to ion-dipole interaction. The dissolution of covalent compounds like urea, glucose and C2H5OH, etc is due to the tendency of these molecules to form hydrogen bond with water.
Chemical Properties of Water
1. Water is amphoteric in nature. It reacts with both acids and bases, and gives salt and water
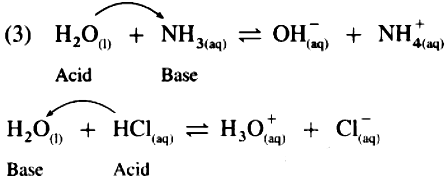
2. In redox reactions, water reacts with metals and non- metals both.
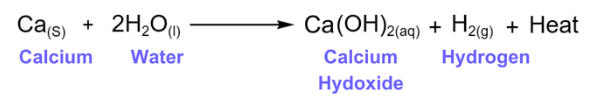 Reaction of Metals with water
Reaction of Metals with water
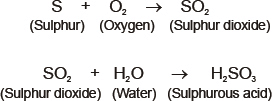 Reaction of non- metals with water
Reaction of non- metals with water
3. In hydrated salts, water may remain in five types such as coordinated water, hydrogen bonded water, lattice water, clathrate water and zeolite water.
4. A number of compounds such as calcium hydride, calcium phosphide, etc., undergo hydrolysis with water.
Purification of Water
It involves two processes:
- Removal of suspended impurities.
- Destroying the bacteria.
Suspended particles are removed by coagulation with alum followed by filtration.
Exposure to sunlight, boiling, chlorination (treatment with liquid Cl2 or bleaching powder), ozonization and addition of CuSO4 are some processes which are employed to destroy bacteria.
Structure of Water
Closely observe the structure of a water molecule. You will see one atom of oxygen and two atoms of hydrogen. Each atom of hydrogen bonds covalently with the atom of oxygen. So both atoms of hydrogen share one pair of electrons with the oxygen atom.
Oxygen is a more electronegative element in comparison to water. This results in an uneven distribution of electron density. This gives the water molecule an angular bent structure. The H-O-H bond has a slight angle of about 104.5°. So it can be said that water molecule is polar. It has a slight negative polarity near the oxygen atom and slight positive charge near both the hydrogen atoms.
Structure of Ice
The structure of the molecules of water in its frozen form i.e. ice is very unique. It forms a Lattice Structure that does not generally occur naturally in any other substance other than ice.
When water reaches its freezing point its atoms rearrange themselves in a very specific three-dimensional pattern. The oxygen atom is surrounded by four hydrogen atoms. Two of these form O-H bonds normally seen in water molecules. The other two form a hydrogen bond.
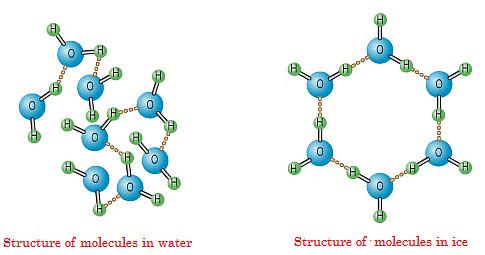
This very special hexagonal shape is what gives ice the unique property of being less dense than water. Since in the structure of ice there are empty spaces between the hexagonal structure, its density is less than that of water in its liquid state. This is why ice floats on water.
Heavy Water (D2O)
It was discovered by Urey in 1932. It can be prepared by exhaustive electrolysis of ordinary water using nickel electrodes. It is colourless, odourless, tasteless liquid.
Chemical Reactions of Heavy Water-
Uses of Heavy Water:
- Used in nuclear reactors to slow down the speed of neutrons and are called moderators.
- Used as a tracer compound to study the mechanisms of many reactions.
Soft and Hard Water
The water which produces large amount of lather with soap is known as soft water and which forms a scum with soap is known as hard water.
Types of Hardness of Water
- Temporary hardness: It is due to the presence of bicarbonates of calcium and magnesium.
- Permanent hardness: It is due to the presence of chlorides and sulphates of calcium and magnesium.
Removal of Temporary Hardness
It can be achieved:(a) By boiling- The soluble bicarbonates are converted into insoluble carbonates.
(b) By Clark’s process- By adding lime water or milk of lime.
[Intext Question]
Removal of Permanent Hardness
(i) By adding washing soda: The calcium or magnesium salts are precipitated as carbonates.
(ii) By adding caustic soda: The temporary and permanent hardness can be removed by adding caustic soda.
(iii) By adding sodium phosphate(Na3PO4): The phosphates of calcium and magnesium are precipitated.
Similarly, magnesium also precipitates out in the form of magnesium phosphate, Mg3(PO4)2.
(iv) Calgon’s process: Calgon is sodium hexa metaphosphate (Na6P6O18). This calgon when added to hard water forms a soluble complex.
Similarly. Mg2+ can also precipitate as Na2[Mg2(PO3)6] and water becomes free from Ca2+and Mg2+ Ions.
(v) Permutit process: Permutit is hydrated sodium aluminium silicate Na2Al2Si2O8.xH2O. It exchanges its sodium ions for divalent ions Ca2+ and Mg2+..
Permutit when fully exhausted can be regenerated by treating with 10% solution of sodium chloride. It is the most efficient method to gel water with zero degree of hardness.
(vi) By synthetic resins:
These are of two types:
(a) Cation exchange resins are big molecules containing sulphonic acid group (-SO3H). It is first changed into sodium salt with the general formula RNa. The hard water is passed through it so Ca2+ and M2+ are exchanged and removed.
The resins like permutit can be regenerated with a solution of NaCl.
(b) Anion exchange resins are also big molecules and can exchange anions. They contain an amino group.
The water is first passed through cation resins and then through anion resins and pure distilled water is obtained.
[Intext Question]
Measurement of Degree of Hardness
Degree of hardness is defined as the number of parts of calcium carbonate or equivalent to various calcium and magnesium salts present in one million parts of water by mass. It is expressed in ppm.
Degree of hardness (in ppm) = (wt. of CaCO3 (g)/ wt. of hard water (g)) x 106
The molecular wt. of Ca(HCO3)2, Mg(HCO3)2, CaCl2, MgCl2, CaSO4 and MgSO4 is 162, 146, 111, 95, 136 and 120 respectively. The mol. wt. of CaCO3 is 100.
Thus, 162 g Ca(HCO3)2, 146 g Mg(HCO3)2, 111 gCaCl2, 95 g MgCl2 136 g CaSO4 and 120 g MgSO4 are equivalent to 100 g CaCO3.
Example: What is the degree of hardness of a sample of water containing 24 mg of MgSO4 (molecular mass 120) per kg of water?
A.10 ppm
B.15 ppm
C.20 ppm
D.25 ppm
Solution: The hardness of water is of two types: permanent and temporary
Temporary hardness is due to the presence of magnesium and calcium hydrogen carbonates.
Presence of insoluble salts like MgSO4 causes permanent hardness of the water.
The amount of hardness causing substances in a certain volume of water measures the degree of hardness.
The hardness of water is always calculated in terms of calcium carbonate (CaCO3). However, it is never responsible for causing hardness as it is insoluble in water.As mentioned, the degree of hardness is expressed as parts per million (ppm) and thus may be defined as the number of parts by weight of CaCO3 (equivalent to Mg salts) present in a million (106) parts by weight of water.
It is given that 1 kg of water contains 24 mg of MgS04. i.e., 1000 g of water contains 24 mg of MgSO4 therefore, 1 million grams of water (106g ) contains 24 mg x 1000 = 24 g of MgSO4
1 mole of MgSO4 = 1 mole of CaCO3The molecular weight of MgS04 = 120g/mol and CaCO3= 100g/mol
120 g of MgSO4 = 100 g 0f CaCO3
Therefore, the equivalent amount of CaCO3 for 24 mg of MgSO4 = 24 x 100/120 = 20 ppm.
Hence, the degree of hardness of water is 20 ppm.
|
334 videos|660 docs|300 tests
|
FAQs on Water: Structure, Properties & Hardness of Water - Chemistry for JEE Main & Advanced
| 1. How does the structure of water molecules contribute to its properties? |  |
| 2. What are the differences between heavy water and normal water in terms of properties? |  |
| 3. How is hard water different from soft water, and what impact does it have on daily life? |  |
| 4. What are some common methods used to soften hard water? |  |
| 5. How can the hardness of water be measured, and what are the recommended levels for domestic use? |  |

















