Wave-Particle Duality - Quantum Mechanics | Physics for IIT JAM, UGC - NET, CSIR NET PDF Download
Wave-Particle Duality
- Wave Particle Duality postulates that matter as well as light exhibits both wave and particle nature.
- This phenomenon is proven not only for elementary particles but for compound particles as well like atoms and molecules.
- In order to explain different phenomena, different assumptions were made about the nature of light. For example, the photoelectric effect of light assumed that light was made up of particles, whereas interference and diffraction assumed that light was made up of waves.
- Wave-particle duality can be used to describe all phenomena. As light was composed of particles or waves, wave-particle dual nature soon was found to be characteristic of electrons as well.
- The particle properties of electrons was well documented when the DeBroglie hypothesis and the subsequent experiments by Davisson and Germer established the wave nature of the electron.
In this document we will discuss how we will discuss how photoelectric effect proved that particle nature of light.
Key Points of Photoelectric Effect
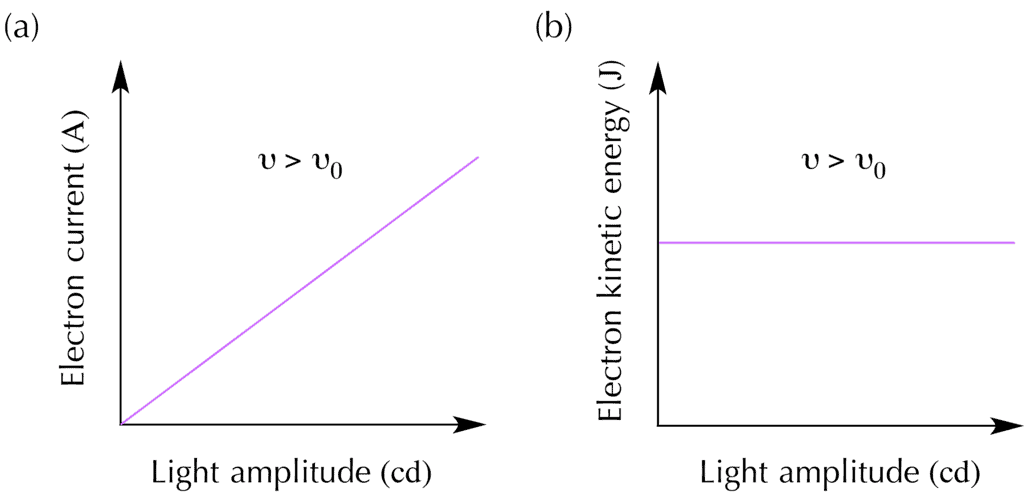
- Based on the wave model of light, physicists predicted that increasing light amplitude would increase the kinetic energy of emitted photoelectrons, while increasing the frequency would increase measured current.
- Contrary to the predictions, experiments showed that increasing the light frequency increased the kinetic energy of the photoelectrons, and increasing the light amplitude increased the current.
- Based on these findings, Einstein proposed that light behaved like a stream of particles called photons with an energy E = hv.
- The work function,Φ, is the minimum amount of energy required to induce photoemission of electrons from a metal surface, and the value of Φ depends on the metal.
- The energy of the incident photon must be equal to the sum of the metal's work function and the photoelectron kinetic energy: Ephoton = KEelectron + Φ.
What is the Photoelectric Effect?
When light shines on a metal, electrons can be ejected from the surface of the metal in a phenomenon known as the photoelectric effect. This process is also often referred to as photoemission, and the electrons that are ejected from the metal are called photoelectrons. In terms of their behavior and their properties, photoelectrons are no different from other electrons. The prefix, photo-, simply tells us that the electrons have been ejected from a metal surface by incident light.
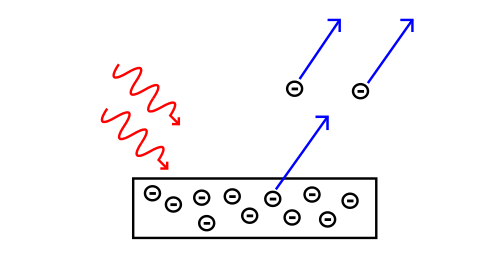 In the photoelectric effect, light waves (red wavy lines) hitting a metal surface cause electrons to be ejected from the metal.
In the photoelectric effect, light waves (red wavy lines) hitting a metal surface cause electrons to be ejected from the metal.
In this document, we will discuss how 19th century physicists attempted (but failed!) to explain the photoelectric effect using classical physics. This ultimately led to the development of the modern description of electromagnetic radiation, which has both wave-like and particle-like properties.
Predictions based on Light as a Wave
- To explain the photoelectric effect, 19th-century physicists theorized that the oscillating electric field of the incoming light wave was heating the electrons and causing them to vibrate, eventually freeing them from the metal surface.
- This hypothesis was based on the assumption that light traveled purely as a wave through space.
- Scientists also believed that the energy of the light wave was proportional to its brightness, which is related to the wave's amplitude.
- In order to test their hypotheses, they performed experiments to look at the effect of light amplitude and frequency on the rate of electron ejection, as well as the kinetic energy of the photoelectrons.
- The kinetic energy of emitted photoelectrons should increase with the light amplitude.
- The rate of electron emission, which is proportional to the measured electric current, should increase as the light frequency is increased.

- If a single large wave were to shake the dock, we would expect the energy from the big wave would send the beach balls flying off the dock with much more kinetic energy compared to a single, small wave. This is also what physicists believed would happen if the light intensity was increased.
- Light amplitude was expected to be proportional to the light energy, so higher amplitude light was predicted to result in photoelectrons with more kinetic energy.
- Classical physicists also predicted that increasing the frequency of light waves (at a constant amplitude) would increase the rate of electrons being ejected, and thus increase the measured electric current.
- Using our beach ball analogy, we would expect waves hitting the dock more frequently would result in more beach balls being knocked off the dock compared to the same sized waves hitting the dock less often.
- Now that we know what physicists thought would happen, let's look at what they actually observed experimentally!
When Intuition Fails: Photons to the Rescue!
- The kinetic energy of photoelectrons increases with light frequency.
- Electric current remains constant as light frequency increases.
- Electric current increases with light amplitude.
- The kinetic energy of photoelectrons remains constant as light amplitude increases.
where Ephoton is the energy of a photon in joules (J), h is Planck's constant (6.626×10-34J⋅s), and ν is the frequency of the light in Hz. According to Planck's equation, the energy of a photon is proportional to the frequency of the light, ν. The amplitude of the light is then proportional to the number of photons with a given frequency.
Light Frequency and the Threshold Frequency
 The frequency of red light (left) is less than the threshold frequency of this metal ( νred < νo ) so no electrons are ejected. The green (middle) and blue light (right) have ( ν > νo ) , so both cause photoemission. The higher energy blue light ejects electrons with higher kinetic energy compared to the green light.
The frequency of red light (left) is less than the threshold frequency of this metal ( νred < νo ) so no electrons are ejected. The green (middle) and blue light (right) have ( ν > νo ) , so both cause photoemission. The higher energy blue light ejects electrons with higher kinetic energy compared to the green light. The scientists observed that if the incident light had a frequency less than a minimum frequency v0, then no electrons were ejected regardless of the light amplitude. This minimum frequency is also called the threshold frequency, and the value of v0 depends on the metal. For frequencies greater than v0, electrons would be ejected from the metal. Furthermore, the kinetic energy of the photoelectrons was proportional to the light frequency. The relationship between photoelectron kinetic energy and light frequency is shown in graph (a) below.

Isn't there more Math somewhere?
Ephoton = KEphoton + Φ
Ephoton = hv = KEphoton + Φ
where me is the rest mass of an electron, 9.1094 x 10-31 kg.
Exploring the Wave Amplitude trends
We are going to discuss how interference provides the proof of wave nature of light in this document.
Questions on Photoelectric Effect
Ephoton = hv
= (6.626 x 10-34 J.s) (3.0 x 1016 Hz) plug in values of h = 2.0 x 10-17 J
If we compare our calculated photon energy to copper's work function, we see that the photon energy is greater than Φ.Thus, we would expect to see photoelectrons ejected from the copper.
Question: According to Einstein’s photoelectric equation, the plot of the kinetic energy of the emitted photoelectrons from a metal vs the frequency of the incident radiation gives a straight line whose slope
(a) depends on the nature of the metal used
(b) depends on the intensity of the radiation
(c) depends both on the intensity of the radiation and the metal used
(d) is the same for all metals and independent of the intensity of the radiation.
Ans.
According to Einstein’s equation, Kinetic energy = hv – Φ where kinetic energy and f (frequency) are variables, compare it with the equation, y = mx + c
Therefore, the slope of line = h, h is Planck’s constant.Hence, the slope is the same for all metals and independent of the intensity of radiation.
FAQs on Wave-Particle Duality - Quantum Mechanics - Physics for IIT JAM, UGC - NET, CSIR NET
| 1. What is the wave-particle duality in quantum mechanics? |  |
| 2. What is the photoelectric effect? |  |
| 3. How does the photoelectric effect support the wave-particle duality? |  |
| 4. What are the practical applications of the photoelectric effect? |  |
| 5. How does the photoelectric effect challenge classical physics? |  |






















