Class 7 Science Chapter 4 Worksheet Solutions - The World of Metals and Non-metals
| Table of contents |

|
| MCQ Questions |

|
| Fill in the Blanks |

|
| True/False Questions |

|
| Very Short Answer Questions |

|
| Long Answer Questions |

|
MCQ Questions
Q1: Which metal is commonly used to make food packaging materials as it is cheaper, and its thin sheets can be folded easily into any shape?
a) Aluminium
b) Copper
c) Iron
d) Gold
Ans: a) Aluminium
Q2: Which of the following metals catches fire when it comes in contact with water?
a) Copper
b) Aluminium
c) Zinc
d) Sodium
Ans: d) Sodium
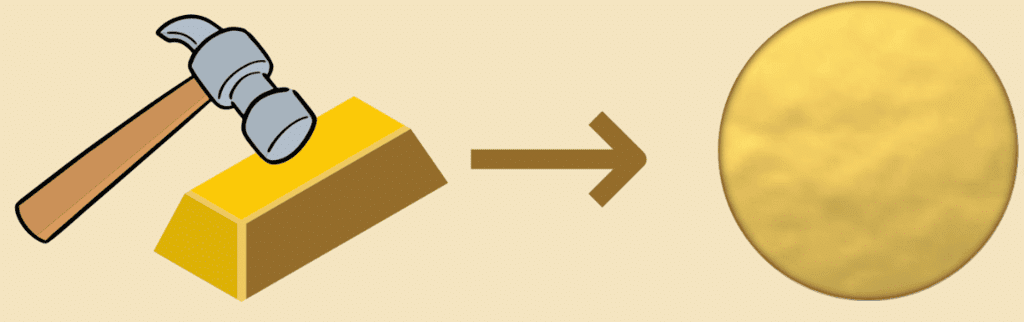
Q3: Which of the following properties do most metals possess?
a) Malleability
b) Dullness
c) Non-conductivity
d) Brittle
Ans: a) Malleability
Q4: Which of these metals is known for being the most malleable and ductile?
a) Gold
b) Copper
c) Iron
d) Aluminium
Ans: a) Gold
Q5: What is the property that enables metals to produce a ringing sound when struck?
a) Malleability
b) Sonority
c) Ductility
d) Lustre
Ans: b) Sonority
Fill in the Blanks
Q1: Materials that allow electricity to flow through them easily are called __________.
Ans: conductors
Q2: The property of metals by which they can be beaten into thin sheets is called __________.
Ans: malleability
Q3: Metals react with oxygen to produce __________ which are basic in nature.
Ans: metal oxides
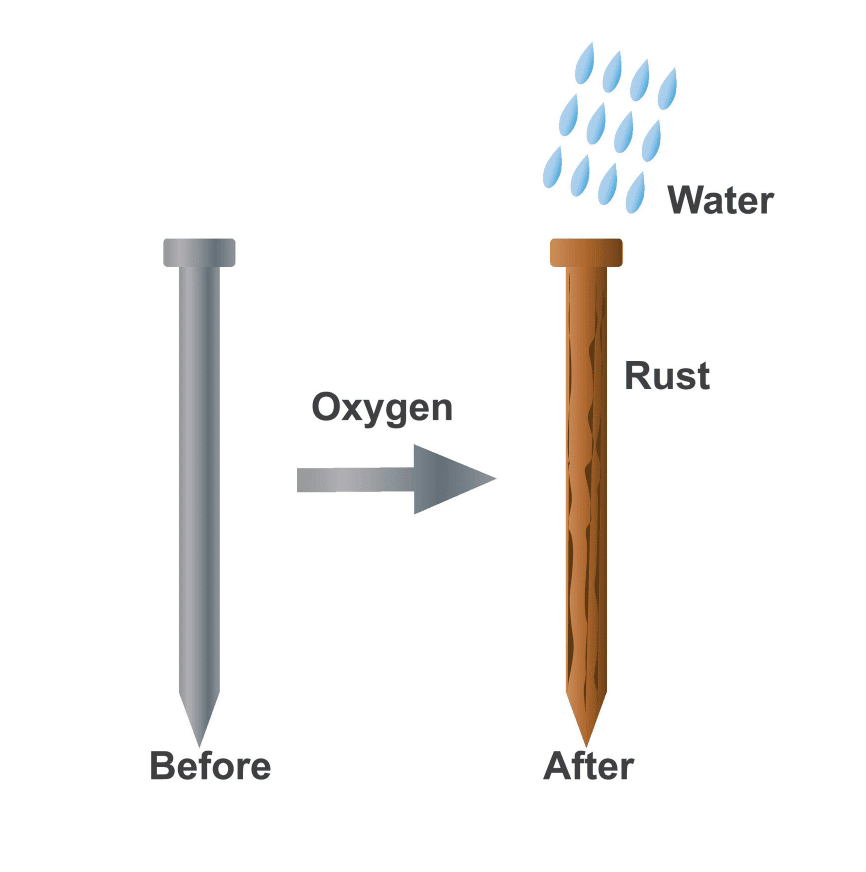
Q4: The process of formation of rust on objects made of iron is called __________.
Ans: rusting
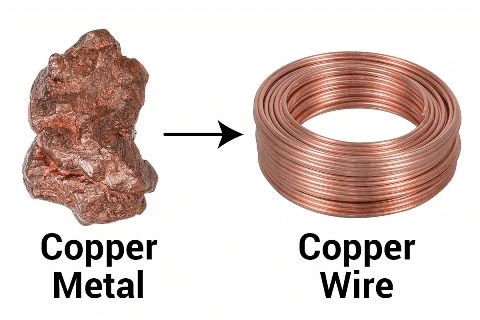
Q5: The property by which materials can be drawn into wires is called __________.
Ans: ductility
True/False Questions
Q1: All metals are hard and lustrous.
Ans: False
Q2: Non-metals are generally poor conductors of heat and electricity.
Ans: True
Q3: Metals do not react with oxygen.
Ans: False
Q4: The rusting of iron can be prevented by applying a protective layer of zinc.
Ans: True
Q5: Magnesium burns with a dazzling white flame and forms a white powder when exposed to oxygen.
Ans: True
Very Short Answer Questions
Q1: What is the process by which metals like iron develop brown deposits?
Ans: This process is called rusting, and it occurs when iron comes into contact with both air and water.
Q2: What is the property of metals that allows them to be beaten into thin sheets?
Ans: Malleability
Q3: Why are materials like copper and aluminium used in electrical wiring?
Ans: Because they are good conductors of electricity.
Q4: What happens when magnesium reacts with oxygen?
Ans: Magnesium burns with a dazzling white flame and forms magnesium oxide
.
Q5: What is the nature of magnesium oxide when dissolved in water?
Ans: Magnesium oxide forms a basic solution when dissolved in water.
Long Answer Questions
Q1: Explain the properties of metals and non-metals and how they differ.
Ans: Metals are generally lustrous, malleable, ductile, and good conductors of heat and electricity. They form basic oxides when they react with oxygen. Non-metals, on the other hand, are usually non-lustrous, brittle, and poor conductors of heat and electricity. They form acidic oxides when they react with oxygen and do not have the properties of malleability and ductility.
Q2: Why are gold and silver used in jewellery, and why are only a few metals suitable for this purpose?
Ans: Gold and silver are highly malleable and ductile, allowing them to be shaped into intricate designs and thin sheets. They are also resistant to corrosion, making them ideal for jewellery. However, not all metals possess these qualities, as some are too hard or reactive to be used for jewellery.
Q3: Describe the process of rusting and the methods used to prevent it.
Ans: Rusting is the process where iron reacts with oxygen and water, forming brown deposits on its surface. To prevent rusting, methods such as painting, oiling, greasing, or applying a protective layer of zinc (galvanisation) are used. These methods prevent exposure to air and water, which are necessary for rust formation.
Q4: How do the properties of metals and non-metals influence their uses in everyday life?
Ans: The properties of metals, such as conductivity, malleability, and ductility, make them useful in applications like electrical wiring, construction, and manufacturing tools. Non-metals, though poor conductors, are essential in biological processes, like oxygen for respiration and carbon for life forms, and are also used in the production of fertilizers and water purification.
Q5: What is the significance of the Iron Pillar of Delhi, and what does it tell us about ancient Indian metallurgy?
Ans: The Iron Pillar of Delhi is a remarkable example of ancient Indian metallurgy. Despite facing exposure to wind, rain, and intense weather for over 1600 years, it shows very little rust. This indicates advanced techniques in metalworking that allowed for resistance to rusting, demonstrating the high level of skill in metallurgy in ancient India.
|
1 videos|107 docs
|
FAQs on Class 7 Science Chapter 4 Worksheet Solutions - The World of Metals and Non-metals
| 1. What are the main differences between metals and non-metals? |  |
| 2. How do metals and non-metals react with acids? |  |
| 3. Can you provide examples of common metals and non-metals? |  |
| 4. What are the uses of metals and non-metals in everyday life? |  |
| 5. How can the reactivity of metals and non-metals be compared? |  |