Class 9 Science Chapter 3 Question Answers - Atoms and Molecules
Q1: Glucose has the molecular formula C6H12O6. Calculate :
(a) Its molecular mass.
(b) The number of atoms in one molecule of glucose.
(c) The number of gram molecule in 18 g of glucose.
Ans:
(a) Molecular mass of C6H12O6
= (6 × 12u) + (12 × 1u) + (6 × 16u)
= 72u + 12u + 96u = 180u
(b) The number of atoms in one molecule of C6H12O6
= 6 atoms of C + 12 atoms of H + 6 atoms of O
= 6 + 12 + 6 = 24 atoms
(c) Number of gram molecules
= Mass of glucose (g) / Molecular mass of glucose (g)
= 18/180
= 0.1
Q2: What is the mass of (i) 2.5 moles of CO2 and (ii) 1 mole of water?
Ans:
(i) 1 mole of CO2 = Molecular mass expressed in grams
= 1 × 44 g 2.5 moles of CO2
= 2.5 × 44 = 110 g
(ii) Mass of the substance = Moles of substance × Molecular mass in grams
Mass of water = 1 × 18 g = 18 g
Q3: Calculate the number of H2O molecules in one drop of water having a mass of 0.05 g.
Ans:
Number of moles of H2O in 0.05 g of water.
=  = (molar mass of water = 18)
= (molar mass of water = 18)
= 
= 1.673 × 1023 molecules
Q4: What is the mass percentage of different elements in calcium carbonate? (Atomic mass : Ca = 40, O = 16)
Ans: Molecular mass of CaCO3 = At. Mass of Ca + At. Mass of C + 3 × At. Mass of O
= 40 + 12 + 3 × 16 = 100
Mass percentage of Ca = 

Q5: What is the use of mole concept?
Ans:
Applications of mole concept :
(i) We can calculate the number of basic particles from the number of moles as the number of moles of a substance is directly proportional to the number of elementary particles.
(ii) One mole of gas occupies 22.4 litres at 273K.
(iii) One mole of any gas occupies the same volume at same pressure and temperature.
(iv) One mole is equal to 6.022 × 1023 atoms. So, we can calculate the absolute masses of atoms and molecules.
Q6: Define the term gram atom. How is it related to mole and Avogadro number?
Ans: The atomic mass of an element expressed in grams is called gram atomic mass. One gram atom of any element contains 6.022 × 1023 atoms of the element. It is equal to one mole of atoms.
One gram atomic mass = 6.022 × 1023 atoms = 1 mole
Q7: Give symbol and valency of : Potassium, Barium, Aluminium, Calcium, Cobalt, Fluorine, Lead, Zinc, Iodine, Sulphide.
Ans:
Q8:Ca2P2O7 is the formula of calcium pyrophosphate. Write the formula for ferric pyrophosphate.
Ans: Valency of calcium is +2. Ca2P2O7 has two calcium atoms. So, calcium have total of +4 charges. Thus, pyrophosphate has a valency of –4. Since ferric ion has a valency of +3, the formula of ferric pyrophosphate is Fe4(P2O7)3-.
Q9: The mass of any single atom X is 3.05 × 10–22 g. What is its atomic weight? Name the possible element.
Ans :
1 mole = atomic mass = 6.022 × 1023 atoms
Now, mass of one atom of X = 3.05 × 10–22 g
Mass of 6.022 × 1023 atoms of X
= 3.05 × 10–22 × 6.022 × 1023 g
= 183.7 g
This element could be tungsten.
Q10: Write formula for the following :
(a) Zinc sulphate,
(b) Methane,
(c) Ammonium carbonate.
Ans:
(a) Zinc sulphate 
Thus, Zn2(SO4)2 and finally = ZnSO4
(b) Methane
Thus, finally = CH4
(c) Ammonium carbonate Thus, finally = (NH4)2CO3
Thus, finally = (NH4)2CO3
Q11: 50 g of 10% lead nitrate is mixed with 50 g of 10% sodium chloride in a closed vessel. It was found after reaction that 6.83 g of lead chloride was precipitated. Besides, the reaction mixture contained 90 g water and sodium nitrate. Calculate the amount of sodium nitrate formed.
Ans:
50 g of 10% lead nitrate = 5 g lead nitrate + 45 g water
50 g of 10% sodium chloride = 5 g sodium chloride + 45 g water
Total content before reaction = 5 + 5 + 90 = 100
Total content after reaction = 90 g
Amount of precipitate = 6.83 g
According to law of conservation,
Total mass of reaction mixture = 100 g
Amount of sodium nitrate = 100 – 90 – 6.83
= 3.17 g
Q12: Explain the law of multiple proportions.
Ans: According to law of multiple proportions, when two elements combine to make one or more compounds then the ratio of weights of these element remain in fixed ratio to one another.
For example: Hydrogen and oxygen combine to form water (H2O) and hydrogen peroxide (H2O2) under different condition. 2 grams of hydrogen combines with 16 grams of oxygen in case of water while 2 grams of hydrogen combines with 32 grams of oxygen to form hydrogen peroxide. Now, the weights of oxygen combine with a fixed weight of hydrogen in water and hydrogen peroxide respectively are 16 and 32 which are in simple ratio of 16: 32 or 1 : 2.
Q13: Explain the form of atoms in a solid.
Ans: A solid element is a cluster of atoms. The property of solid does not depend on a single atom but on cluster of atoms.
For example : Diamond and graphite though both are composed of carbon atoms but due to different arrangements of carbon atoms in these. They are different in physical and chemical properties.
Q14: What are molecules? Give brief explanation of the arrangement of the constituent atoms in the molecules.
Ans: A molecule is the smallest particle of an element or compound which is stable in normal conditions. And it can freely show all the properties of that element or compound. It may be made up of one, two or more atoms. Molecule with one atom called monoatomic. E.g. helium, neon, etc.
Molecule with two atoms called diatomic. E.g. Cl2, O2. Similarly, there are molecules containing three atoms (CO2), four atoms (P4) and so on.
Q15: The mass of one molecule of a substance is 4.65 × 1023 grams. What is its molecular mass?
Ans: Mass of 1 molecule of a substance = 4.65 × 1023 grams
Mass of 6.023 × 10–23 molecules of a substance
= 4.65 × 1023 × 6.023 × 10–23
= 28 g
Molecular mass of the substance = 28 g
Q16: Chlorine occurs in nature in two isotopic forms with masses 35u and 37u in the ratio of 3 : 1. What should be the mass of chlorine atom?
Ans:
= 142 / 4
= 35.5u
Q17: An element 12X24 loses two electron to form a cation which combines with the anion of element 17Y35 formed by gaining an electron.
(a) Write the electronic configuration of element X.
(b) Write the electronic configuration of the anion of element Y.
(c) Write the formula for the compound formed by combination of X and Y.
Ans:
(a) X = 2, 8, 2
(b) Y– = 2, 8, 8
(c) XY2
Q18: Calculate the formula unit masses of ZnO, Na2O, K2CO3 given atomic masses of Zn = 65u, Na = 23u, K = 39u, C = 12u, and 0 = 16u.
Ans:
Formula unit mass of ZnO = 1 × 65u + 1 × 16u
= 81u
Formula unit mass of Na2O = 2 × 23u + 1 × 16u
= 62u
Formula unit mass of K2CO3 = 2 × 39u + 1 × 12u + 3 × 16u
= 138u
Q19: If one mole of carbon atoms weighs 12 grams, what is the mass (in gram) of 1 atom of carbon?
Ans: 1 mole of carbon weighs = 12 g
1 atom of carbon weighs = 
= 1.99 × 10–23 g
Q20: What is the mass of :
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminium atoms (atomic mass of aluminium = 27)?
(c) 10 moles of sodium sulphite (Na2SO3)?
Ans:
(a) 1 mole of nitrogen atoms
= 1 × gram atomic mass of nitrogen atom
= 1 × 14 g = 14 g
(b) 4 moles of aluminium atoms
= 4 × gram atomic mass of aluminium atoms
= 4 × 27 g = 108 g
(c) 10 moles of sodium sulphite (Na2SO3)
= 10 (2 × gram atomic mass of Na + 1 × gram atomic mass of sulphur + 3 × gram atomic mass of oxygen)
= 10 (2 × 23 g + 1 × 32 g + 3 × 16g)
= 10 (46 g + 32 g + 48 g)
= 10 × 126 g = 1260 g
Q21: Give the postulates of Dalton’s atomic theory.
Ans: Every element is composed of extremely small particles called atoms. Atoms of a given element are identical, both in mass and properties. Different chemical elements have different kinds of atoms; in particular, their atoms have different masses.
Atoms cannot be created, destroyed or transformed into atoms of other elements. Compounds are formed when atoms of different elements combine with each other in small whole number ratios. The relative number and kinds of atoms in a given compound are constant.
Q22: (a) Give one point of difference between an atom and an ion.
(b) Give one example each of a polyatomic cation and an anion.
(c) Identify the correct chemical name of FeSO3 : Ferrous sulphate, Ferrous sulphide, Ferrous sulphite.
(d) Write the chemical formula for the chloride of magnesium.
Ans:
(a) An atom is electrically neutral while an ion is electrically charged particle.
(b) (i) Polyatomic cation : (NH4)+ (ii) Polyatomic anion : (SO4)2–
(c) Ferrous sulphite
(d) MgCl2 (Magnesium chloride)
Q23: When 3.0 g of magnesium is burnt in 2.00 g of oxygen, 5.00 g of magnesium oxide is produced. What mass of magnesium oxide will be formed when 3.00 g magnesium is burnt in 5.00 g of oxygen? Which law of chemical combination will govern your answer? State the law.
Ans:
When 3.0 g of magnesium is burnt in 2.00 g of oxygen, 5.00 g of magnesium oxide is produced. It means magnesium and oxygen are combined in the ratio of 3 : 2 to form magnesium oxide.
Thus, when 3.00 g of magnesium is burnt in 5.00 g of oxygen, 5.00 g of magnesium oxide will be formed and the remaining oxygen will be left unused. It is governed by law of definite proportions.
It states that in a chemical substance, the elements are always present in definite proportions by mass.
Q24: (a) Calculate the number of molecules of SO2 present in 44 g of it.
(b) If one mole of oxygen atoms weighs 16 grams, find the mass of one atom of oxygen in grams.
Ans:
(a) Molecular mass of SO2 = Atomic mass of S + 2 × Atomic mass of O
= 32 + 2 × 16
= 64u
Molar mass = 64 g
Number of molecules, N = 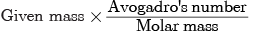
= 44/64 x 6.022 x 1023
= 4.14 × 1023 molecules
(b) One mole of oxygen contains 6.022 × 1023 atoms of oxygen
Mass of one atom of oxygen = 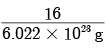
= 2.66 × 10–23 g
Q25: Sodium is represented as 23Na11.
(a) What is its atomic mass?
(b) Write its gram atomic mass.
(c) How many atoms of Na will be there in 11.5 g of the sample?
Ans:
(a) Atomic mass = 23u
(b) Gram atomic mass = 23 g
(c) Given mass = 11.5 g
Molar mass = 23 g Number of atoms (N) = 
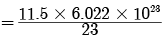
= 3.011 × 1023 atoms
|
84 videos|384 docs|61 tests
|





















