NCERT Solutions for Class 7 Science - Physical and Chemical Changes
Exercise
 Q1. Classify the changes involved in the following processes as physical or chemical changes:
Q1. Classify the changes involved in the following processes as physical or chemical changes:
(a) Photosynthesis
Ans: Chemical change
 View Answer
View Answer 
Photosynthesis is a process in which plants convert carbon dioxide and water into glucose and oxygen using sunlight. This process results in the formation of new chemical substances (glucose and oxygen), making it a chemical change.
(b) Dissolving sugar in water
Ans: Physical change
 View Answer
View Answer 
When sugar dissolves in water, it mixes evenly, but its chemical composition remains the same. No new substance is formed, and it can be separated by evaporation or crystallization, making it a physical change.
(c) Burning of coal
Ans: Chemical change
 View Answer
View Answer 
When coal burns, it reacts with oxygen to form carbon dioxide and ash. This results in new substances with different chemical properties, making it a chemical change.
(d) Melting of wax
Ans: Physical change
 View Answer
View Answer 
Melting is a change of state from solid to liquid. The chemical composition of wax remains the same; only its form changes. Therefore, it is a physical change.
(e) Beating aluminium to make aluminium foil
Ans: Physical change
 View Answer
View Answer 
When aluminium is beaten into thin sheets, only its shape and size change, but its chemical composition remains the same. Therefore, it is a physical change.
(f) Digestion of food
Ans: Chemical change
 View Answer
View Answer 
Digestion involves the breakdown of complex food molecules into simpler substances using enzymes. New chemical substances (nutrients and waste products) are formed. Therefore, it is a chemical change.
Q2. State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change. (True/False)
Ans: False
 View Answer
View Answer 
Cutting a log of wood into pieces is a physical change because only the size and shape of the wood change, but its chemical composition remains the same.
(b) Formation of manure from leaves is a physical change. (True/False)
Ans: False
 View Answer
View Answer 
Formation of manure from leaves is a chemical change because decomposition takes place, leading to the formation of new substances.
(c) Iron pipes coated with zinc do not get rusted easily. (True/False)
Ans: True
 View Answer
View Answer 
Zinc coating protects iron from rusting through a process called galvanization, as zinc forms a protective layer that prevents iron from coming in contact with moisture and oxygen.
(d) Iron and rust are the same substances. (True/False)
Ans: False
 View Answer
View Answer 
Iron and rust are different substances. Rust is iron oxide,which is formed when iron reacts with oxygen and moisture.
(e) Condensation of steam is not a chemical change. (True/False)
Ans: True
 View Answer
View Answer 
Condensation is a physical change because water vapor changes back to liquid water without any change in its chemical composition.
Q3. Fill in the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of _________.
Ans: Calcium carbonate.
 View Answer
View Answer 
Lime water (Ca(OH)₂) reacts with carbon dioxide (CO₂) to form calcium carbonate (CaCO₃), which is insoluble in water and appears as a milky white precipitate.
(b) The chemical name of baking soda is _________.
Ans: Sodium hydrogen carbonate.
 View Answer
View Answer 
Baking soda is commonly used in cooking and as an antacid. Its chemical formula is NaHCO₃, and it is also known as sodium bicarbonate.
(c) Two methods by which rusting of iron can be prevented are _________ and _________.
Ans: Polishing, Galvanisation.
 View Answer
View Answer 
- Painting: Creates a protective layer that prevents iron from coming in contact with air and moisture.
- Galvanisation: Coating iron with zinc prevents rusting because zinc forms a protective layer and does not corrode easily.
(d) Changes in which only _________ properties of a substance change are called physical changes.
Ans: Physical
 View Answer
View Answer 
In a physical change, only the state, shape, or size of a substance changes, but its chemical composition remains the same. Examples include melting, freezing, dissolving, and cutting.
(e) Changes in which new substances are formed are called _________ changes.
Ans: Chemical
 View Answer
View Answer 
In a chemical change, one or more new substances with different properties are formed. These changes are usually irreversible. Examples include burning, rusting, digestion, and photosynthesis.
Q4. When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Ans: When baking soda is mixed with lemon juice, bubbles form because of the evolution of carbon dioxide gas. This is a chemical change because, in this reaction, new substances are formed.
Lemon juice + Baking soda → Carbon dioxide + other substances
Q5. When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Ans: In the burning of a candle, some of the wax melts, which is a physical change, while most of the wax burns, which is a chemical change.
In the burning of wood, water present in wood changes into a vapour; this is a physical change, while burning of wood is a chemical change.
Q6. How would you show that the setting of curd is a chemical change?
Ans: In the setting of curd, milk changes into a new substance, curd. This is an irreversible process which means milk cannot be retrieved from the curd. The process of curd setting does involve chemical changes due to fermentation.
Q7. Explain why burning wood and cutting it into small pieces are considered two different types of changes.
Ans: In the burning of wood, new substances are formed and hence, there is a chemical change. While cutting the wood into small piece,s no new substance is formed, thus it is a physical change.
Hence, burning wood and cutting it into small pieces are considered two different types of changes.
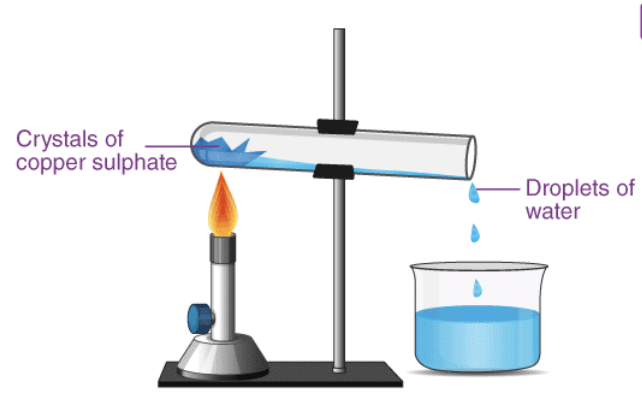
Q8. Describe how crystals of copper sulphate are prepared.
Ans: Process:
- Fill a beaker with water.
- Add a few drops of dilute sulphuric acid to the water.
- Heat the water until it starts to boil.
- Gradually add copper sulphate powder while stirring the solution continuously.
- Once copper sulphate begins to settle at the bottom, filter the solution.
- Allow the solution to cool undisturbed.
- After cooling, crystals of copper sulphate will form.
Q9. Explain how painting of an iron gate prevents it from rusting.
Ans: Iron rusts due to a reaction with oxygen in moist air. Painting an iron gate helps prevent rusting by:
- Creating a barrier that stops oxygen from contacting the iron.
- Reducing exposure to moisture, which accelerates rust formation.
- Providing a protective layer that can be reapplied as needed.
Regular maintenance of the paint is essential to ensure effective protection against rust.
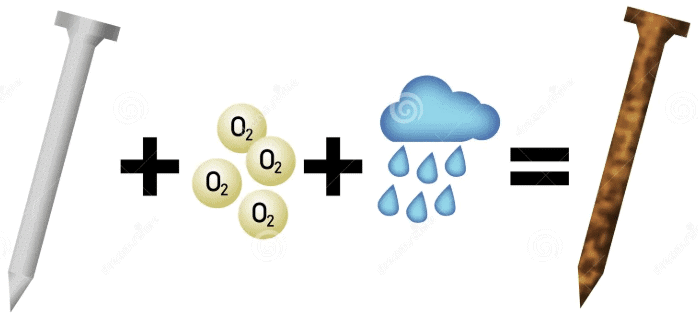
Q10. Explain why rusting of iron objects is faster in coastal areas than in deserts.
Ans: Rusting of iron objects occurs more rapidly in coastal areas than in deserts due to several factors:
- The presence of moisture in coastal air is higher because of nearby seas or oceans.
- Rusting requires both oxygen and water; thus, more humid conditions accelerate the rusting process.
- In contrast, desert air is typically dry and hot, which slows down rusting.
Therefore, iron objects corrode faster in coastal regions compared to arid deserts.
Q11. The gas we use in the kitchen is called liquified petroleum gas (LPG). In the cylinder it exist as a liquid. When it comes out from the cylinder, it becomes a gas (Change – A), then it burns (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Ans: (ii) Process – B is a chemical change.
 View Answer
View Answer 
- Process A, where liquefied petroleum gas (LPG) changes from a liquid to a gas, is a physical change.
- Process B, where the gas burns, is a chemical change.
Q12. Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change.
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Ans: (iii) Both processes A and B are chemical changes.
 View Answer
View Answer 
- Process A involves anaerobic bacteria breaking down animal waste, which is a chemical change.
- Process B is the burning of biogas, which is also a chemical change.
|
111 videos|286 docs|28 tests
|
FAQs on NCERT Solutions for Class 7 Science - Physical and Chemical Changes
| 1. What are the main differences between physical changes and chemical changes? |  |
| 2. Can you provide examples of physical changes and chemical changes? |  |
| 3. How can we identify a chemical change? |  |
| 4. Are physical changes reversible? |  |
| 5. Why is it important to understand physical and chemical changes in everyday life? |  |

















