Class 8 Science Chapter 11 Question Answers - Chemical Effects of Electric Current
Q1: Differentiate between good conductors and bad conductors of electricity.
Ans:
| Good conductors of electricity | Bad conductors of electricity |
|
|
Q2: Describe an electrical tester.
Ans: An electrical tester is a simple piece of electronic test equipment used to determine the presence or absence of an electric voltage in a piece of equipment under test. It is also used to test whether a liquid allows electric current to pass through it or not.
Q3: How can we check whether a tester is working or not?
Ans: Join the free ends of the tester together, for a moment, and check whether the bulb glows or not, if the bulb glows it means tester is working and if not it means tester is not working. In case it does not glow check that all connections are tight, or not.
Q4: How can you test whether lemon juice is good conductor or poor conductor of electricity?
Ans: Pour one table spoon of lemon juice in a plastic cap of discarded bottle, dip the end of tester into lemon juice, make sure that the tester should not more than 1 cm apart at also should not touch each other, we will observe the current flows in the circuit and bulb glows, this proves that lemon juice is good conductor.
Q5: Why the filament of bulb does not get heated sometimes in a circuit?
Ans: This is because current through the circuit is too weak so the filament of the bulb does not get heated sufficiently and it does not glow.
Q6: Is it possible for an electric tester to detect weak current also, if no how can we detect weak current flowing in a circuit?
Ans: If weak current flows through the circuit then bulb in it will not glow. In order to detect weak current we use LED or may use another effect of electric current that is it produces magnetic effect also so we can use this property of electric current to detect weak current.
Q7: Why materials classified as poor conductors, also allow electricity to pass under certain conditions?
Ans: Under certain condition most of the materials can conduct electricity thus it is right to say poor conductors instead of bad conductors or insulators.
Q8: Distilled water is good conductor or bad, how can we make distilled water a good conductor?
Ans: Distilled water is a poor conductor but if we add some salt in it the resulting salt solution is good conductor.
Q9: Explain the functioning of a LED.
Ans: LED stands for Light emitting diode. It is a semiconductor light source, use to detect weak current in the circuit. There are two wires attached to a LED, one lead is slightly longer than the another one, the longer lead is always connected to the positive terminal of battery and shorter lead is connected to negative terminal of battery.
Q10: What are the purpose of one longer and another shorter lead of a LED?
Ans: the longer lead is always connected to the positive terminal of battery and shorter lead is connected to negative terminal of battery.
Q11: Why tap water is always good conductors?
Ans: Tap water is not pure, it contains several amounts of mineral salts naturally dissolved in it , thus it is good conductor.
Q12: Why water that we get from ponds and hand pumps are always good conductors?
Ans: Water that we get from ponds and hand pumps is not pure, it contains several amounts of mineral salts naturally dissolved in it , thus it is good conductor.
Q13: We add small amount of dilute hydrochloric acid to distilled water, the resulting solution will be a good conductor or poor conductor? Explain why.
Ans: Good conductor, because when we add kitchen salt (NaCl) to the distilled water the salt dissolves in water by splitting its molecules in ions:
H2O + NaCl→ H2O + (Na+) + (Cl-)
The NaCl molecules react to give ions. This happens because the NaCl is a strong electrolyte. Solutions of strong electrolytes are good conductors of electricity because they contain a relatively high concentration of ions.
Q14: We add small amount of sugar to distilled water, the resulting solution will be a good conductor or poor conductor?Explain why.
Ans: Poor conductor, because Sugar, as a non-electrolyte substance, does not produce ions when dissolved in water. A solution of sugar contains molecules of sucrose,but no ions. The absence of ions in a sugar aqueous solution makes it a non-electricity conductor fluid.
Q15: What happens when electrodes are immersed in water and a current is passed?
Ans: When electrodes are immersed in water and a current is passed bubbles of oxygen and hydrogen is produced.
Q16: Explain the process of electroplating.
Ans: One of the most common applications of the chemical effect of electric current is electroplating. In this process, there exists a liquid, usually called the electrolyte, through which current passes. Two electrodes, connected to the terminals of a battery with a switch in between, are inserted in the liquid. The electrode that is connected to the positive terminal of the battery is called the “anode,” and the other connected to the negative terminal is called the “cathode”. Electroplating is done in industries to have an anti-reactive coating on the parts of machines so that they do not react with the raw material, to have an anti-corrosive coating for the machines so that they do not get corroded, and a heat-resistive coating for parts like boilers to resist the heat produced by the machinery.The process of electroplating is used for plating parts of vehicles with nickel and chromium, which protects them from corrosion.
Q17: What do you mean by gold plating?
Ans: Gold plating is one of the most common applications of electroplating in ornament-making.
Q18: What do you mean by electrolysis and electrolytes?
Ans: Electrolysis: The passage of an electric current through a liquid causes chemical changes. This process is known as electrolysis.
Electrolytes: Conduction is possible only in those liquids which are at least partly dissociated into oppositely charged ions; such liquids are called electrolytes.Solutions of many inorganic chemical compounds (e.g. common salt, sulphuric acid, etc.) are examples of this type of liquid.
Q19: What do you mean by voltameter?
Ans: In electrolysis, the whole arrangement of electrodes, electrolyte and the vessel containing them is called a voltameter. In the case of the copper voltameter, which involves copper electrodes in copper sulphate solution, the net effect is that copper is dissolved off the anode and deposited on the cathode, with the electrolyte remaining unchanged.
Q20: When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain why?
Ans: The deflection through the compass needle shows that current is flowing through the wounded wire and hence through the circuit. The circuit is complete since free ends of the tester are dipped in the solution and the solution is certainly conducting solution.
Q21: In case of fire before the fireman used the water hoses, they shut off the main electrical supply of the area, why so?
Ans: Water may conduct electricity, if the electrical supply of the area is not shut off and water is poured over electrical appliances then electricity may pass through fire and may harm the fireman, thus In case of fire before the fireman used the water hoses, they shut off the main electrical supply of the area.
Q22: In which case the compass needle will deflect more while testing sea water or while testing drinking water?
Ans: The compass needle will defect more in case of sea water than drinking water because sea water contains more dissolved salt than the drinking water.
Q23: Differentiate between conductors and insulators.
Ans: The substances which allow the electric current to pass through them are called conductors. Example metals like copper, aluminium. The substances which do not allow electric current to pass through them are called insulators, example : rubber, wood.
Q24: Write some of the chemical effects of electric current. What causes the chemical reactions in a conducting solution?
Ans: The passage of an electric current through a conducting solution causes chemical reactions. Following are listed some of the chemical effects of electric current:
1. Bubbles of a gas may be formed on the electrodes.
2. Deposits of metal may be seen on electrodes.
3. Changes of colour of solutions may occur.
4. Infact, the reaction would depend on what solution and electrodes are used.
Q25: Is it safe for an electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Ans: No, it is not at all safe for an electrician to carry out electrical repairs during heavy downpour. Rather, it is highly dangerous, because during heavy downpour there is a high risk of electrocution.
Q26: Can you prepare an electric pen? Describe how?
Ans: Yes we can prepare an electric pen by performing following experiment.
- Take a filter paper and soak it with potassium iodide and starch solution.
- Spread this filter paper on a metal sheet.
- Now take two connecting wires and join them to the terminals of battery.
- Attach the wire connected at positive end to plate.
- Now write on the sheet wire attached to negative terminal.
We will see that wherever we write, blue coloured ink will appear. The reason for this is electrolysis of potassium iodide solution which produces iodine. Iodine on reaction with starch produces ink of blue colour.
Q27: What do you mean by electric current?
Ans. The continuous and directional flow of charges (electrons) is called electric current. It is denoted by I and its unit is ampere.
Q28: What is a tester?
Ans. The instrument which is used to check the flow of electric current is called tester. It is attached to the terminals of the electric circuit. If the bulb of tester glows, it confirms that current is flowing through the circuit.
Q29: How can you test whether the liquids conduct or do not conduct the electricity?
Ans. Some liquids are the good conductors of electricity while some are poor conductors. The liquids can be tested for conductor of electricity. The free ends of a tester are dipped in liquid to be tested and then observe the bulb, if it glows, it confirms that the liquid is good conductor otherwise liquid is a poor conductor.
Q30: Show that lemon juice and vinegar are good conductors of electricity.
Ans. Collect a few plastic or rubber caps of bottles. Pour one teaspoon of lemon juice or vinegar in one cap. Bring the tester over the cap and let the ends of the tester dip into lemon juice or vinegar. We see that bulb starts to glow. It indicates that lemon juice and vinegar are good conductors of electricity.
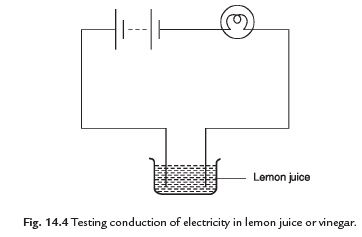
Q31: Explain the mechanism of glowing of bulb in liquid.
Ans. When the liquid between the two ends of a tester allows the electric current to pass, the circuit of the tester becomes complete. The current flows in the liquid circuit and the bulb glows. When the liquid does not allow the electric current to pass, the circuit of the tester is not complete and the bulb does not glow.
Q32: There are some situations in which even though liquid is conducting, bulb may not glow. Give reasons.
Ans. The possible reasons may be:
(i) The current may be weak.
(ii) Bulb may be fused.
(iii) Incomplete circuit.
Q33: What is LED? Why is it most important source of light?
Ans. The device which is used in the tester in place of bulb is called LED. It glows even at very small current. There are two wires called leads attached to the LED. One lead is longer than the other. A long wire is connected with the positive terminal and shorter lead is connected to the negative terminal of battery.
Q34: What do you mean by magnetic effect of electricity?
Ans. When electric current is passed through a coil or wire, then it behaves like a magnet. This is called magnetic effect of current. The strength of magnetic field depends on the amount of current passing through a coil or wire. The coil or wire shows magnetism till current is passed.
Q35: The ordinary water can conduct electricity while distilled water does not. Explain why.
Ans. The water that we get from various sources like taps, hand pumps, wells and ponds is not pure. It may contain several salts dissolved in it. This water is thus good conductor of electricity. Distilled water is free of salts due to which it is a poor conductor.
Q36: Why do we need magnetic compass to test the conduction of electric current?
Ans. Sometimes the bulb does not glow on passing electric current. This is because the electric current flowing through a conductor is so small, that the filament of the bulb does not get heated up to the temperature where it starts glowing. So, in case of small current we need magnetic compass to test the conduction.
Q37: What is chemical effect of electricity? Give some examples of chemical effects.
Ans. The process in which a chemical reaction or change takes place in a solution on passing electricity is called chemical effect of electricity.
The passage of an electric current through a conducting solution causes chemical reactions. For example, change in colour of solutions and electroplating.
|
90 videos|296 docs|44 tests
|
FAQs on Class 8 Science Chapter 11 Question Answers - Chemical Effects of Electric Current
| 1. What are the chemical effects of electric current? |  |
| 2. How does an electric current cause the decomposition of electrolytes? |  |
| 3. What is electrolysis? |  |
| 4. What factors affect the chemical effects of electric current? |  |
| 5. Give an example of a chemical effect of electric current. |  |

















