Introduction to biomass management, Biomass Management for Fodder & Energy | Biomass Management for Fodder & Energy - Agricultural Engineering PDF Download
Introduction
With serious concern globally and in India on the use of fossil fuels, it is important for India to start using renewable energy sources. India is the seventh largest country in the world spanning 328 million hectares and amply bestowed with renewable sources of energy. Among the renewable energy sources, biomass plays a vital role especially in rural areas, as it constitutes the major energy source to majority of households in India. India produces about 450-500 million tonnes of biomass per year. Biomass provides 32% of all the primary energy use in the country at present.
1.2. Biomass
Biomass is defined as the organic matter derived from biological materials such as plants, animals, microorganisms and municipal wastes. It is a renewable form of energy as it can be replenished within a short period. It can be used as a solid fuel, or converted into liquid or gaseous forms for the production of electric power, heat, chemicals or fuels.
As per the Energy Independence and Security Act of 2007, the term “renewable biomass” means each of the following:
(i) Planted crops and crop residue harvested from agricultural land cleared or cultivated at any time prior to the enactment of this sentence that is either actively managed or fallow, and non-forested.
(ii) Planted trees and tree residue from actively managed tree plantations on non-federal land cleared at any time prior to enactment of this sentence, including land belonging to an Indian tribe or an Indian individual, that is held in trust by the United States or subject to a restriction against alienation imposed by the United States.
(iii) Animal waste material and animal byproducts.
(iv) Slash and pre-commercial thinning that are from non-federal forestlands, including forestlands belonging to an Indian tribe or an Indian individual, that are held in trust by the United States or subject to a restriction against alienation imposed by the United States, but not forests or forestlands that are ecological communities with a global or State ranking of critically imperiled, imperiled, or rare pursuant to a State Natural Heritage Program, old growth forest, or late successional forest.
(v) Biomass obtained from the immediate vicinity of buildings and other areas regularly occupied by people, or of public infrastructure, at risk from wildfire.
(vi) Algae.
(vii) Separated yard waste or food waste, including recycled cooking and trap grease.
1.3. Reasons for utilizing biomass
Readily available and renewable
Non-fossil forms of fixed carbon are not depletable, in contrast to fossil fuels such as coal, oil, petroleum fuels and natural gas.
Biomass is available in large quantities and provides a raw material for conversion to major supplies of synthetic fuels
Combining waste disposal and energy recovery processes offers recycling opportunities as well as improved disposal technology, often at low cost.
Clean and nearly pollution free combustion
Energy and capital requirement for production is low
1.4. Biomass management
The biomass can be converted to useful secondary energy forms such as heat, gaseous fuels, solid fuels, organic chemical and liquid fuels. There are several alternative routes for producing useful secondary energies from biomass. Biomass conversion is surely the solution not only to reduce our dependence on fossil fuels but also to solve the problem of agricultural residues. It is important to say that biomass absorbs the same amount of CO2 in growing that it releases when burned as a fuel in any form. This means that biomass contribution to global warming is zero.
1.5. Biomass management technologies
Biomass conversion may be carried on two broad pathways:
Thermo chemical conversion and
Biochemical conversion
Chemical conversion
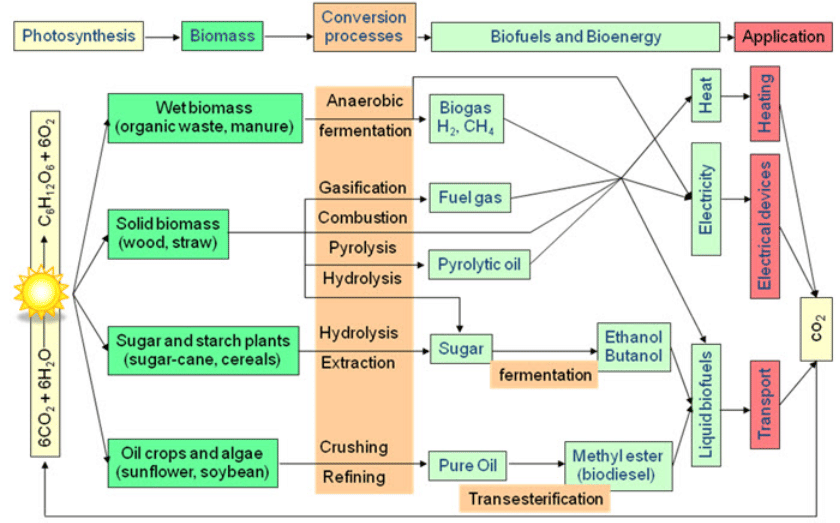
Biomass conversion chart
1.6. Biomass characteristics
The ease and efficiency with which biomaterials can be converted to energy are largely determined by their physicochemical properties. There is no standardized method for the characterization of biomaterial with respect to its potential for conversion to energy for fuels. In the case of thermo-chemical conversion processes, proximate analysis, ultimate analysis and heating value are important parameters. In the case of biochemical conversion processes, the amount and chemical form of the carbohydrate constituents of the biomaterials are important parameters.
1.6.1. Proximate Analysis
The proximate analysis characterizes the material in terms of its moisture, volatile matter, ash, and by difference "fixed" carbon content. The proximate analysis gives the percentage of material burned in the gaseous state (volatile matter) and in the solid state (fixed carbon) as well as an indication of the amount of ash residue.
Proximate Analysis of some Biomass materials
Fuel type | Volatile matter % | Ash % | Fixed carbon % |
Rice husk IR-3 | 68.60 | 17.40 | 14.00 |
Patnai-23 | 69.30 | 15.80 | 14.90 |
Padma | 68.90 | 18.60 | 12.70 |
Arhar stalks | 82.90 | 1.98 | 15.12 |
Pigeon pea | 79.20 | 4.94 | 15.86 |
Cotton sticks | 8140 | 3.30 | 15.30 |
Dhaincha stalks | 82.70 | 2.98 | 14.32 |
Groundnut shell | 83.90 | 4.43 | 11.67 |
Maize stalks | 79.57 | 3.36 | 17.07 |
Maize cobes | 83.01 | 1.83 | 15.16 |
Rice Straw | 69.70 | 19.20 | 11.10 |
Wheat straw | 73.60 | 8.47 | 17.93 |
1.6.2. Ultimate Analysis
The ultimate analysis involves elemental analyses for carbon, hydrogen, nitrogen, sulfur, and by difference, oxygen. The ultimate analysis is used to calculate the chemical balance of the combustion reactions as well as the quantity of combustion air and excess air required. Additionally, the ultimate analysis enables identification and quantification of the potential pollutants resulting from the thermo-conversion of fuels.
1.6.3. Heating value or energy Content
The energy content of biomass (heat of combustion) is usually determined by use of a bomb calorimeter, which measures the energy change for combustion to gaseous carbon dioxide and water vapour. This gives the "higher" or "gross" heating value of the biomass (HHV), including energy recovered from the condensation of the water.
1.7. Thermo chemical conversion technologies
Thermo-chemical conversion technologies can be utilized for energy conversion of low moisture herbaceous and woody biomass. The three routes of thermo chemical conversion of biomass are:
Pyrolysis
Gasification and
Combustion
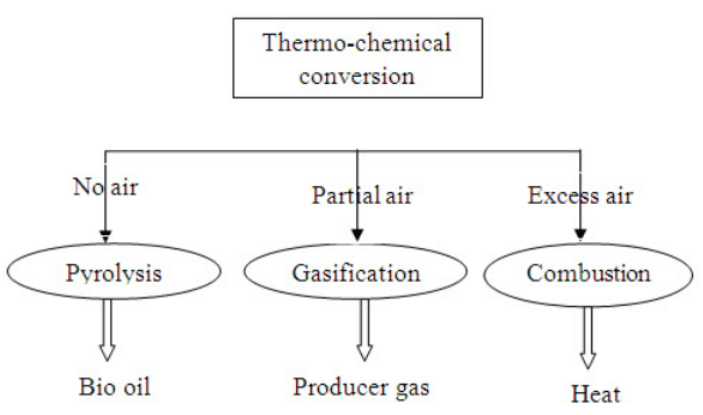
1.7.1. Pyrolysis
Pyrolysis is the process of heating biomass in the absence of oxygen. The products of biomass pyrolysis are charcoal, oils and tars, water and permanent gases including methane, hydrogen, carbon-mono oxide and Carbon dioxide. The nature of the changes in pyrolysis depend on the material being pyrolyzed, the final temperature of the process and the rate at which it is heated up (slow or fast pyrolysis).
Typically the slow pyrolysis is conducted for hours to a maximum temperature of 400oC - 500oC. The charcoal yield is 35% to 40% by weight. The goal of fast pyrolysis is to produce liquid fuel (bio oil or pyrolytic oil) from biomass that can substitute for fuel oil in any application. Bio oil can also be used to produce a range of specialty and commodity chemicals. The essential features of a fast pyrolysis process are very high heating and heat transfer rates, which often require a finely ground biomass feed.
1.7.2. Combustion
Combustion is a process in which the fuel is burnt with oxygen from the air to release the stored chemical energy as heat in burners, boilers, internal combustion engines and turbines. It is the most direct process of biomass conversion into energy that can be used for a variety of applications such as cooking, process heating, power generation and cogeneration. In order to harness biomass energy to the maximum extent, it is important to understand biomass combustion. This includes understanding properties of biomass fuels and the fundamentals of numerous complex reactions associated with biomass combustion.
Combustion is a process whereby the carbon and hydrogen in the fuel react with oxygen ultimately to form carbon dioxide and water through a series of free radical reactions resulting in the liberation of heat. The overall combustion reaction can be written as
CxHyOz + (x + 0.25y - 0.5z)O2→ xCO2 + (0.5y).H2O
The combustion efficiency is mainly determined by the completeness of the combustion process. The moisture content of biomass, excess air and the flame temperature plays an important role in deciding the overall efficiency of the combustion.
1.7.3. Gasification
Gasification is a thermo chemical transformation of a biomass by partial oxidation into a gaseous product. The reactions are carried out at elevated temperatures, 500-1400oC, and atmospheric or elevated pressures up to 33 bar. The oxidant used can be air, pure oxygen, steam or a mixture of these gases.
Pyrolysis is only one of the steps in the conversion process. The other steps are combustion with air and reduction of the products of combustion (water vapour and carbon dioxide) into combustible gases (carbon monoxide, hydrogen, methane and some higher hydrocarbons) and inert gases (carbon dioxide and nitrogen). The end result is producer gas, having calorific value 950 -1200 kcal/m3, which can be used in internal combustion engines with some fine dust and condensable compounds termed tar, both of which must be restricted to less than about 100 ppm each. The producer gas obtained by the process of gasification can have end use for thermal application or for mechanical /electrical power generation.
A few of the major reactions involved in gasification of biomass are:
Exothermic Reactions:
(1) C + O2 → CO2+393800 kJ/kg mol (Combustion reaction)
(2) C + 2H2→ CH4+ 75000 kJ/kg mol (Methanation reaction)
(3) CO + H2O→ CO2 + H2+41200 kJ/kg mol (Water gas shift reaction)
Endothermic Reactions:
(4) C + H2O → CO + H2 – 131400 kJ/kg mol (Water gas reaction)
(5) C + CO2 → 2CO – 172600 kJ/kg mol (Boudouard reaction)
(6) C + 2H2O→ CO2 + 2H2 – 78700 kJ/kg mol
1.8. Biochemical conversion
The biochemical routes of conversion of biomass are essentially anaerobic digestion and fermentation.
1.8.1. Anaerobic digestion
Anaerobic digestion is a type of biochemical conversion involving the microbial digestion of biomass in the absence of air. An anaerobe is a microscopic organism that can live and grow without external oxygen or air. It extracts oxygen by decomposing the biomass at low temperatures up to 65°C, in presence of moisture (80%). It produces biogas which is a mixture of methane 55-65% and CO2 35-45% and some impurities such as hydrogen sulphide in traces. The gas can be burned directly or upgraded to superior fuel gas (methane) by removing the CO2 and impurities. The residue of the anaerobic digestion may consist of protein-rich sludge and liquid effluents. These can be used as animal feed or for soil treatment after certain processing.
In general, one kg of dry organic material will produce 0.036 m3 of methane (at standard temperature and pressure) or 36 m3 biogas/1000 kg biomass. The sizes of anaerobic digestion plants vary from 0.5 m3/ day to 2000 m3/day. In India anaerobic digestion plants are commonly known as biogas plants or gobar gas plants. In such plants slurry of cow dung and water is fed to the digester and is allowed to ferment for a few weeks. The biogas is released. The gas is being used in villages for cooking, lighting, running diesel engines and fuel for furnaces etc.
Anaerobic digestion technologies are being widened for using feedstocks such as
urban (municipal) waste, agricultural biomass (straw of rice, wheat, sugar cane bagasse etc.), forest biomass (trees, leaves), aquatic biomass (algae, water-plants) and human and animal excreta.
1.8.2. Fermentation
The fermentation is a process of decomposition of organic matter by microorganisms especially bacteria and. yeasts. Examples of fermentation include decomposition of grains, sugar to form ethyl alcohol (ethanol) and carbon dioxide by yeast (in making of wine) and ethyl alcohol forming acetic acid (in making vinegar). About 15% of ethanol produced in the world is through fermentation of grains and molasses.
Ethanol (Ethyl Alcohol) can be blended with gasoline (petrol) to produce gasohol
(90% petrol and 10% ethanol). Processes have been developed to produce various fuels from various types of fermentations. Ethanol fermentationof biomass occurs at 20 to 30°C. The process takes about 50 hours. Yield is about 90% liquid. This contains about 10 to 20% of alcohol depending upon the tolerance of yeast to alcohol. Concentration of alcohol is increased by distillation.















