JEE Exam > JEE Notes > Additional Study Material for JEE > Intermolecular Interactions
Intermolecular Interactions | Additional Study Material for JEE PDF Download
Intermolecular Forces
- Intermolecular forces can be described as the forces of attraction and repulsion existing between the interactingparticles of atoms and molecules in a compound.
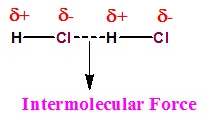
- The attractive intermolecular forces are called van der Waals forces which include dispersion forces or London forces, dipole-dipole forces, and dipole-induced dipole forces.
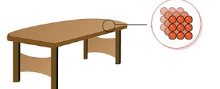
- Intermolecular forces in case of solids are very strong. The constituent particles are closely packed thereby making the solids incompressible and of high density.
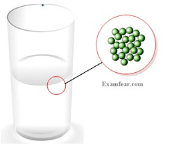
- Intermolecular forces in case of liquids keeps the particles tied to each other but cannot keep them in fixed positions thereby making them easy to flow and have a definite shape.
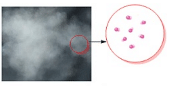
- Intermolecular forces in case of gases are extremely weak thereby allowing the constituent particles to move freely.
The document Intermolecular Interactions | Additional Study Material for JEE is a part of the JEE Course Additional Study Material for JEE.
All you need of JEE at this link: JEE
|
22 videos|163 docs|17 tests
|
FAQs on Intermolecular Interactions - Additional Study Material for JEE
| 1. What are intermolecular interactions? |  |
Ans. Intermolecular interactions are the forces of attraction between molecules. These interactions play a crucial role in determining the physical and chemical properties of substances. They can be classified into various types, such as hydrogen bonding, dipole-dipole interactions, London dispersion forces, and ion-dipole interactions.
| 2. How do intermolecular interactions affect the boiling point of a substance? |  |
Ans. Intermolecular interactions directly influence the boiling point of a substance. Substances with strong intermolecular forces, such as hydrogen bonding, require more energy to overcome these forces and change from a liquid to a gas phase. Hence, they have higher boiling points compared to substances with weaker intermolecular forces.
| 3. Can intermolecular interactions affect the solubility of a substance? |  |
Ans. Yes, intermolecular interactions significantly impact the solubility of a substance. For instance, polar solutes tend to dissolve in polar solvents due to the formation of favorable dipole-dipole interactions. On the other hand, nonpolar solutes dissolve better in nonpolar solvents due to London dispersion forces. The compatibility of intermolecular forces between the solute and solvent determines the solubility.
| 4. How do intermolecular interactions affect the surface tension of a liquid? |  |
Ans. Intermolecular interactions influence the surface tension of a liquid. Surface tension is a measure of the force required to increase the surface area of a liquid. Strong intermolecular forces lead to higher surface tension as the molecules at the surface are more tightly held together. Substances with weaker intermolecular forces have lower surface tension.
| 5. Can intermolecular interactions affect the viscosity of a liquid? |  |
Ans. Yes, intermolecular interactions have a significant impact on the viscosity of a liquid. Viscosity is a measure of a liquid's resistance to flow. Substances with strong intermolecular forces, such as hydrogen bonding, have higher viscosity as the molecules are tightly held together, impeding their movement. In contrast, substances with weaker intermolecular forces have lower viscosity as the molecules can move more freely.
Related Searches
















