Henderson equation: Calculation of pH of a Buffer Mixture | Chemistry for JEE Main & Advanced PDF Download
For Acidic Buffer mixture( Henderson- Hasselbalch equation)
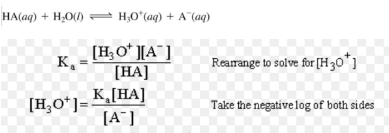
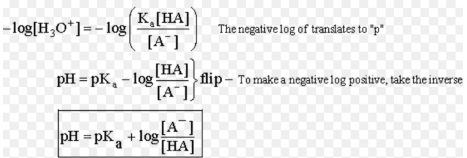
For Basic Buffer mixture
pOH = pKb + log [salt] / [Base]
pH + pOH =14
pOH = 14 – pH
pKa + pKb = 14
pKb=14 – pKa
14 – pH = 14 – pKa + log [salt) / [base]
pH = pKa – log [salt] / [base]
pH = pKa + log [base] / [salt]
where Ka is the ionization constant of the conjugate acid of the base.
For ex: In the buffer NH4OH + NH4Cl , NH4+ is the conjugate acid of the base, NH3and Ka represents the ionization constant of the reaction:
NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq)
pH = pKa + log [NH3] / [NH4+]
pH = pKa + log [Base] / [conjugate acid]
Buffer Capacity
It is defined as the number of moles of an acid or a base required to be added to one litre of the buffer solution so as to change its pH by one unit.
Buffer Capacity = No. of moles of the acid or base added to 1 litre of the buffer / change in pH
Buffer capacity of a buffer is maximum when the concentration of the weak acid and its salt or weak base and its salt are equal i.e. when pH = pKa or pOH = pKb
Importance of Buffer Solution
1) In biological processes
The pH of our blood is maintained constant inspite of various acid and base producing reactions going on in our body.The buffer action is due to the presence of carbonic acid , bicarbonate ion and carbon dioxide in the blood.
2) In industrial processes
The use of buffers is an important part of many industrial processes, e.g.,
in electroplating, in the manufacture of leather, dyes, photographic materials.
3) In analytical chemistry
1) in the removal of acid radicals such as phosphate, oxalate and borate which interfere in the precipitation of radicals of group 3.
2) in complexometric titration
3) to calibrate the pH metres
4)In bacteriological research, culture media are generally buffered to maintain pH required for the growth of the bacteria being studied.
|
361 videos|822 docs|301 tests
|
FAQs on Henderson equation: Calculation of pH of a Buffer Mixture - Chemistry for JEE Main & Advanced
| 1. How is the Henderson equation used to calculate the pH of a buffer mixture? |  |
| 2. What is the significance of a buffer mixture in maintaining pH? |  |
| 3. How does the Henderson equation relate to acid-base equilibrium? |  |
| 4. Can the Henderson equation be used for any buffer mixture? |  |
| 5. How can the Henderson equation be applied in practical scenarios? |  |
















