JEE Advanced (Fill in the Blanks): Structure of Atom | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The mass of a hydrogen atom is ............. kg. (1982 - 1 Mark)
Ans. 1.66 × 10–27 Kg
Sol. 1.66 × 10–27 Kg
Mass of hydrogen atom
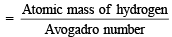
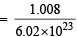
= 0.166 × 10–23 g = 1.66 × 10–27 kg
Q.2. Isotopes of an element differ in the number of ............. in their nuclei. (1982 - 1 Mark)
Ans. neutrons
Sol. neutrons
Q.3. When there are two electrons in the same orbital, they have ............. spins. (1982 - 1 Mark)
Ans. antiparallel or opposite
Sol. antiparallel or opposite
Q.4. Elements of the same mass number but of different atomic numbers are known as ............... . (1983 - 1 Mark)
Ans. isobars
Sol. isobars
Q.5. The uncertainty principle and the concept of wave nature of matter were proposed by ............... and ............... respectively. (Heisenberg, Schrodinger, Maxwell, de Broglie) (1988 - 1 Mark)
Ans. Heisenberg, de-Broglie
Sol. Heisenberg, de-Broglie
Q.6. The light radiations with discrete quantities of energy are called ............... . (1993 - 1 Mark)
Ans. Photons
Sol. Photons
Q. 7. Wave functions of electrons in atoms and molecules are called ............... . (1993 - 1 Mark)
Ans. oribtals
Sol. oribtals
Q.8. The 2px ,2py and 2pz orbitals of atom have identical shapes but differ in their ............... . (1993 - 1 Mark)
Ans. orientation in space
Sol. orientation in space
Q. 9. The outermost electronic configuration of Cr is ............... . (1994 - 1 Mark)
Ans. 3d5 4s1
Sol. 4s1, 3d5;
The electronic configuration of Cr is : 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5.
∴ Outermost electronic configuration is 3d5, 4s1.
|
481 docs|964 tests
|





















