JEE Advanced (Matrix Match & Integer Answer): Structure of Atom | Chapter-wise Tests for JEE Main & Advanced PDF Download
Each question contains statements given in two columns, which have to be matched. The statements in Column-I are labelled A, B, C and D, while the statements in Column-II are labelled p, q, r, s and t. Any given statement in Column-I can have correct matching with ONE OR MORE statement(s) in Column-II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example :

If the correct matches are A-p, s and t; B-q and r; C-p and q; and D-s then the correct darkening of bubbles will look like the given.
Q.1. According to Bohr ’s theory, (2006 - 6M) En = Total energy, Kn = Kinetic energy, Vn = Potential energy, rn = Radius of nth orbit Match the following :

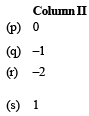
Ans. Sol. (A) - (r); (B) - (q); (C) - (p); (D) - (s)
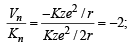 where
where 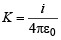 ∴ (i) – (c)
∴ (i) – (c)
(ii) 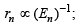 ∴ (ii)-(b)
∴ (ii)-(b)
(iii) Angular momentum of electron in lowest (1s) orbital
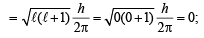 ∴ (iii) (a )
∴ (iii) (a )
(iv) 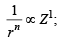 ∴ (iv)- (d)
∴ (iv)- (d)
Q.2. Match the entries in Column I with the correctly related quantum number(s) in Column II. Indicate your answer by darkening the appropriate bubbles of the 4 × 4 matrix given in the ORS (2008 - 6M)
Column I Column II
(A) Orbital angular momentum of the electron in a (p) Principal quantum number hydrogen-like atomic orbital
(B) A hydrogen-like one-electron wave function (q) Azimuthal quantum number obeying Pauli principle
(C) Shape, size and orientation of hydrogen- like (r) Magnetic quantum number atomic orbitals
(D) Probability density of electron at the nucleus (s) Electron spin quantum number in hydrogen-like atom
Ans. Sol. A-q,r; B-p,q,r,s; C-p, q, r; D-p, q
|
481 docs|964 tests
|
















