JEE Advanced (Fill in the Blanks): Chemical Bonding & Molecular Structure | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The angle between two covalen t bonds is maximum in ................. . (CH4, H2O, CO2) (1981 - 1 Mark)
Ans. Sol. CO2; Bond angle in CH4 is 109°.28’, in H2O it is 105 and in CO2 it is 180°. So it is maximum in case of CO2
Q.2. Pair of molecules which forms strongest intermolecular hydrogen bond is ................. .
(SiH4 and SiF4,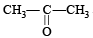 and CHCl3,
and CHCl3, 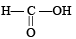 and
and 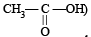 (1981 - 1 Mark)
(1981 - 1 Mark)
Ans. Sol. 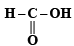 and
and 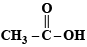 form strongest hydrogen bonds because of largest difference in electronegativities of bonded atoms.
form strongest hydrogen bonds because of largest difference in electronegativities of bonded atoms.
Q.3. There are ............. π bonds in a nitrogen molecule. (1982 - 1 Mark)
Ans. Sol. 2; N ≡ N (N2) has 1σ and 2π bonds. (A triple bond consists of 1σ and 2π bonds)
Q.4. ............. hybrid orbitals of nitrogen atom are involved in the formation of ammonium ion. (1982 - 1 Mark)
Ans. Sol. sp3; Hybridisation (H)= 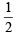 [No. of valence electron in centralatom + No. of monovalent atoms – Charge on cation + Charge on anion]
[No. of valence electron in centralatom + No. of monovalent atoms – Charge on cation + Charge on anion]
For N in NH4+, hybridisation (H) = 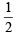 (5 + 4 – 1 + 0) = 4
(5 + 4 – 1 + 0) = 4
∴ sp3 hybridisation.
Q.5. The shape of [CH3]+ is ............... . (1990 - 1 Mark)
Ans. Sol. Planar; +CH3 is a carbocation and such a species has aplanar shape.
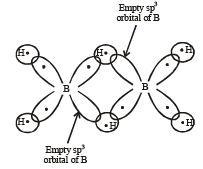
Q.6. The two types of bonds present in B2 H6 are covalent and ........ (1994 - 1 Mark)
Ans. Sol. Three centred two electron bonds or banana bond;
NOTE : The formation of three centred two electron bond is due to one empty sp3 orbital of one of the B atom, 1s orbital of the bridge hydrogen atom and one of the sp3 (filled) orbital of the other B-atom. This forms a delocalized orbital covering the three nuclei giving the shape of a banana. Thus also known as banana bonds.
Q.7. When N2 goes to N+2 , the N–N bond distance ..., and when O2 goes to O+2 the O–O bond distance.... (1996 - 1 Mark)
Ans. Sol. Increases, decreases;
∴ Bond order in N2 = 3 and Bond order in N2+ = 2.5
Thus conversion of N2 to N+2 decreases bond order (from 3 to 2.5) and hence increases the N – N bond distance. [∵ Bond distance increases with decrease in B.O.)
Bond order in O2 = 2 and Bond order in O+2 = 2.5
NOTE : Thus conversion of O2 to O+2 increases bond order (from 2 to 2.5) hence decrease O – O bond distance.
|
481 docs|964 tests
|
















