JEE Advanced (Fill in the Blanks): Chemical Kinetics & Nuclear Chemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Fill in the Blanks
1. An element 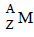 undergoes an α-emission followed by two successive β-emissions. The element formed is ............. . (1982 - 1 Mark)
undergoes an α-emission followed by two successive β-emissions. The element formed is ............. . (1982 - 1 Mark)
Ans: 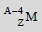
Solutions :
TIPS/Formulae :
When an element emits α -particle atomic mass decreases by four and atomic number decreases by two. Loss of β - particle results in increase in atomic number by 1 and no
change in atomic mass.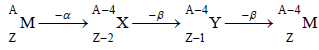
2. The rate of chemical change is directly proportional to ............... . (1985 - 1 Mark)
Ans: Product of active masses of reactants at that temperature
Solutions :
Product of active masses of reactants at that time
3. The number of neutrons in the parent nucleus which gives 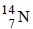 on beta emission is ............... . (1985 - 1 Mark)
on beta emission is ............... . (1985 - 1 Mark)
Ans: 8
Solutions : 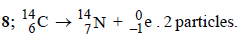
4. The hydrolysis of ethyl acetate in ............... medium is a ............... order reaction. (1986 - 1 Mark)
Ans: acidic, first (or basic, second)
5. A radioactive nucleus decays emitting one alpha and two beta particles; the daughter nucleus is ............... of the parent. (1989 - 1 Mark)
Ans: isotope
Solutions : Isotope; (because new atom has same atomic number but different atomic mass).
6. For the reaction N2 (g) + 3H2 (g) → 2NH3 (g), under certain conditions of temperature and partial pressure of the reactants, the rate of formation of NH3 is 0.001 kg h–1. The rate of conversion of H2 under the same condition is........kg h–1.
Ans: 1.765 × 10–4 kg/hr
Solutions : N2(g) + 3H2(g) —→ 2NH3(g)
Here Rate of reaction = 1/3 [Rate of disappearance of H2] = 1/2 [Rate of appearance of NH3]
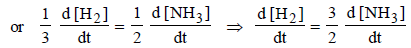
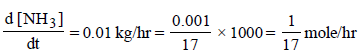
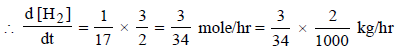
= 1.765 × 10–4 kg/hr.
7. In the Arrhenius equation, 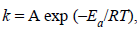 A may be termed as the rate constant at .............. . (1997 - 1 Mark)
A may be termed as the rate constant at .............. . (1997 - 1 Mark)
Ans: very high temperature or zero activation energy
Solutions : very high temperature (T=∞) or zero activation energy
|
446 docs|929 tests
|





















