Integer Answer Type Questions: Chemical Kinetics & Nuclear Chemistry | JEE Advanced | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Integer Value Correct Type
1. The total number of a and b particles emitted in the nuclear reaction 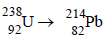
Ans: 8
Solution : 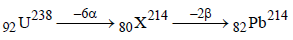
Hence total number of particles emitted are 2 +6 = 8
2. The concentration of R in the reaction R → P was measured as a function of time and the following data is obtained:
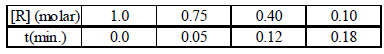
The order of reaction is (2010)
Ans: 5
Solution : The integrated form of a zero-order reaction is
[A0] – [At] = k0t ; 1.0 – 0.75 = k0 × 0.05, k0 = 5
Again, 1.0 – 0.4 = k0 × 0.12, k0 = 5
3. The number of neutrons emitted when 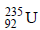 undergoes controlled nuclear fission to
undergoes controlled nuclear fission to 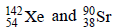 is (2010)
is (2010)
Ans: 3
Solution : 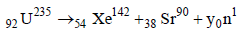
235 = 142 + 90 + y Þ y = 3.
The number of neutrons emitted are 3.
4. An organic compound undergoes first-order decomposition. The time taken for its decomposition to 1/8 and 1/10 of its initial concentration are t1/8 and t1/10 respectively. What is the value of 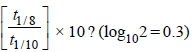 (2012)
(2012)
Ans: 9
Solution : 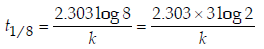
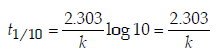
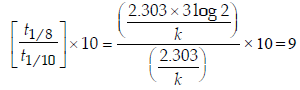
5. The periodic table consists of 18 groups. An isotope of copper, on bombardment with protons, undergoes a nuclear reaction yielding element X as shown below. To which
group, element X belongs in the periodic table? (2012)
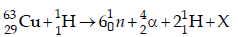
Ans: 8
Solution : 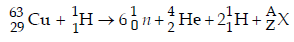
Balancing the atomic mass and atomic number
63 + 1 = (6 × 1) + 4 + 2 + A ⇒ A = 52
29 + 1 = (6 × 0) + 2 + 2 + Z ⇒ Z = 26
Thus, 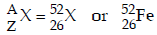
Hence, X belongs to group 8 in the periodic table.
6. A closed vessel with rigid walls contains 1 mol of 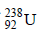 and 1 mol of air at 298 K. Considering complete decay of
and 1 mol of air at 298 K. Considering complete decay of 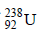 to
to 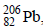 , the ratio of the final pressure to the initial pressure of the system at 298 K is (JEE Adv. 2015)
, the ratio of the final pressure to the initial pressure of the system at 298 K is (JEE Adv. 2015)
Ans: 9
Solution : Number of moles in gas phase, at start (ni) = 1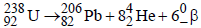
Now number of moles in gas phase, after decomposition (nF)
= 1 + 8 = 9 mole
at constant temperature and pressure
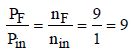
7. In dilute aqueous H2SO4, the complex diaquodioxalatoferrate(II) is oxidized by 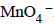 . For this reaction, the ratio of the rate of change of [H+] to the rate of change of
. For this reaction, the ratio of the rate of change of [H+] to the rate of change of
Ans: 8
Solution :
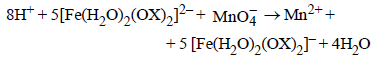
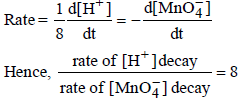
|
347 docs|185 tests
|
FAQs on Integer Answer Type Questions: Chemical Kinetics & Nuclear Chemistry - JEE Advanced - 35 Years Chapter wise Previous Year Solved Papers for JEE
| 1. What is the rate determining step in chemical kinetics? |  |
| 2. How is the rate of a chemical reaction determined? |  |
| 3. What is activation energy in chemical kinetics? |  |
| 4. What are the factors that affect the rate of a chemical reaction? |  |
| 5. What is nuclear chemistry and why is it important? |  |
















