JEE Advanced (Fill in the Blanks): The s-Block Elements | Chapter-wise Tests for JEE Main & Advanced PDF Download
Fill in the Blanks
Q.1. Anhydrous MgCl2 is obtained by heating hydrated salt with ..........
Ans. Anhydrous HCl
Solution. Anhydrous HCl
∵ All the water of crystallisation cannot be removed by heating hydrated MgCl2.
Q.2. The absorption of hydrogen by palladium is commonly known as ............... .
Ans. occlusion
Solution. occlusion.
Q.3. Sodium dissolved in liquid ammonia conducts electricity because ............... .
Ans. of solvated electrons
Solution. of solvated electrons.
Q.4. The electrolysis of molten sodium hydride liberates ............. gas at the ............. .
Ans. Hydrogen, anode
Solution.
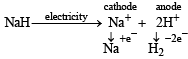
Q.5. Ca2+ has a smaller ionic radius than K+ because it has ............
Ans. higher effective nuclear charge
Solution. higher effective nuclear charge.
True / False
Q.1. MgCl2.6H2O on heating give anhydrous MgCl2.
Ans. F
Solution. Although 4 molecules of water of crystallisation are removed by heating, the remaining two react with MgCl2 as per the equation given below :
MgCl2 + 2H2O → MgO + 2HCl + H2O
NOTE : In order to avoid this to happen, MgCl2.2H2O is dehydrated in presence of HCl gas, which checks, (being in excess) the hydrolysis of MgCl2 by its own water of crystallisation.
Q.2. The softness of group I-A metals increases down the group with increasing atomic number.
Ans. T
Solution. The metallic bonding decreases with increase in atomic size and thus the tendency to show metallic bonding among alkali metals decreases from Li to Cs and thus close packing of atoms in crystal lattice decreases from Li to Cs resulting in an increase in softness.
Q.3. Sodium when burnt in excess of oxygen gives sodium oxide.
Ans. F
Solution. Sodium when burnt in excess of oxygen gives monoxide and sodium peroxide (Na2O2) and not sodium oxide.
4Na + O2 → 2Na2O; 2Na + O2 → Na2O2
|
446 docs|929 tests
|
















