JEE Advanced (Matrix Match): The d & f-Block Elements & Coordination Compounds | Chapter-wise Tests for JEE Main & Advanced PDF Download
DIRECTIONS (Q. No. 1 and 2) : Each question contains statements given in two columns, which have to be matched. The statements in Column-I are labelled A, B, C and D, while the statements in Column-II are labelled p, q, r, s and t. Any given statement in Column-I can have correct matching with ONE OR MORE statement(s) in Column-II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example :
If the correct matches are A-p, s and t; B-q and r; C-p and q; and D-s then the correct darkening of bubbles will look like the given.

Q.1. Match the complexes in Column I with their properties listed in Column II.
Column I Column II
(A) [Co(NH3)4(H2O)2]Cl2 (p) geometrical isomers
(B) [Pt(NH3)2Cl2] (q) paramagnetic
(C) [Co(H2O)5Cl]Cl (r) diamagnetic
(D) [Ni(H2O)6]Cl2 (s) metal ion with +2 oxidation state
Ans. (A - p, q, s); (B - p, r, s); (C - q, s); (D - q, s)
Solution. In [Co(NH3)4(H2O)2]2+, Co is in + 2 state having 3d7 configuration, which makes it paramagentic due to odd electrons. Moreover, it is an octahedral complex showing cis-trans isomerism w.r.t., H2O.
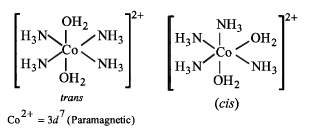
(B) : (p), (r) and (s)
In [Pt (NH3)Cl2], Pt is in + 2 state with configuration 5d8.
Since NH3 is a strong field ligand, it will pair all the electrons making the complex diamagnetic. Moreover, it is a square planar complex showing cis-trans isomerism.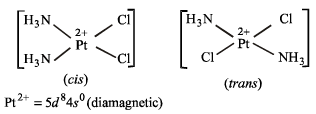
(C) : (q) and (s) In [Co(H2O)5Cl]Cl, Co is in + 2 state with 3d7 configuration making it paramagnetic.
(D) : (q) and (s) In [Ni(H2O)6]Cl2, Ni is in + 2 state with 3d8 configuration. It is attached with weak field ligands, therefore it is paramagnetic.
Q.2. Match each of the reactions given in Column I with the corresponding product(s) given in Column II.
ColumnI Column II
(A) Cu + dil HNO3 (p) NO
(B) Cu + conc HNO3 (q) NO2
(C) Zn + dil HNO3 (r) N2O
(D) Zn + conc HNO3 (s) Cu (NO3)2
(t) Zn(NO3)2
Ans. (A - p, s); (B - q, s); (C - r, t); (D - q, t)
Solution.
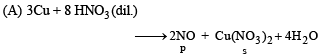
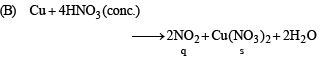
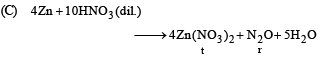
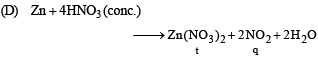
DIRECTIONS (Q. No. 3) : Following question has matching lists. The codes for the list have choices (a), (b), (c) and (d) out of which ONLY ONE is correct.
Q.3. Match each coordination compound in List-I with an appropriate pair of characteristics from List- II and select the correct answer using the code given below the lists.
{en = H2NCH2CH2NH2; atomic numbers : Ti = 22; Cr = 24; Co = 27; Pt = 78}
List-I List-II
P. [Cr(NH3)4Cl2]Cl 1. Paramagnetic and exhibits ionisation isomerism
Q. [Ti(H2O)5Cl](NO3)2 2. Diamagnetic and exhibits cis-trans isomerism
R. [Pt(en)(NH3)Cl]NO3 3. Paramagnetic and exhibits cis-trans isomerism
S. [Co(NH3)4(NO3)2]NO3 4. Diamagnetic and exhibits ionisation isomerism
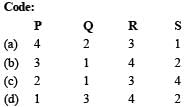
Ans. (b)
Solution.
Complex | Magnetic character | Isomerism |
P, [Cr(NH3)4Cl2]Cl | Cr3+ is d3, hence paramagnetic | cis-trans |
Q, [Ti(H2O)5Cl](NO3)2 | Ti3 + is d1, hence paramagnetic. | ionization |
R, [Pt(en)(NH3)Cl]NO3 | Pt 2+ is d8, complex is square planar, all electrons are paired, hence diamagnetic | ionization |
S, [Co(NH3)4(NO3)2]NO3 | Co3+ is d6, all electrons are paired due to strong ligands, hence diamagnetic | cis-trans |
|
446 docs|929 tests
|





















