JEE Advanced (Subjective Type Questions): The p-Block Elements- 1 | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Account for th e followin g. Limit your an swer to two sentences
(i) Hydrogen bromide cannot be prepared by action of concentrated sulphuric acid or sodium bromide.
(ii) When a blue litmus paper is dipped into a solution of hypochlorous acid, it first turns red and then later gets decolourised. (1979)
Solution. (i) HBr is a reducing agent and it reduces H2SO4 to SO2.
(ii) Acids turn blue litmus red, so HClO also turns blue litmus red. The colour of litmus is decolourised because HClO is also a strong oxidising agent.
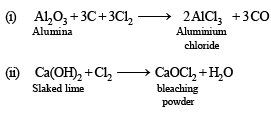
Q.2. Write balanced equation involved in the preparation of
(i) Anhydrous aluminium chloride from alumina.
(ii) Bleaching powder from slaked lime.
(iii) Tin metal from cassiterite
(iv) Chlorine from sodium chloride.
(v) Nitric oxide from nitric acid.
Solution.
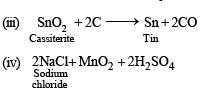
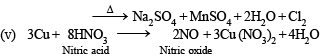
Q.3. State with balanced equations, what happens when :
(i) Tin is treated with moderately concentrated nitric acid.
(ii) Aluminium is reacted with hot concentrated caustic soda solution
Solution.
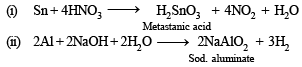
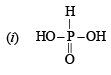
Q.4. Give structural formula for the following :
(i) Phosphorous acid, H3PO3 (1981 - 1 Mark)
(ii) Pyrophosphoric acid, H4P2O7 (1981 - 1 Mark)
Solution.
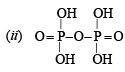
Q.5. Complete the following equations (no balancing is needed)
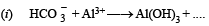 (1981 - 1 Mark)
(1981 - 1 Mark)
(ii) AlBr3 + K2Cr2O7 + H3PO4 —→ K3PO4 + AlPO4 + H2O +....+.... (1981 - 1 Mark)
Solution.
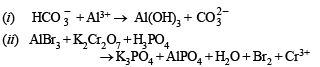
Q.6. Give reasons for the following :
(i) Carbon acts as an abrasive and also as a lubricant. (1981 - 1 Mark)
(ii) Sulphur melts to a clear mobile liquid at 119ºC, but on further heating above 160ºC, it becomes viscous. (1981 - 1 Mark)
(iii) In the preparation of hydrogen iodide from alkali iodides, phosphoric acid is preferred to sulphuric acid (1982 - 1 Mark)
(iv) Orthophosph or ic acid, H3PO4, is tribas ic, but phosphorous acid, H3PO3, is dibasic. (1982 - 1 Mark)
(v) A bottle of liquor ammonia should be cooled before opening the stopper. (1983 - 1 Mark)
(vi) Solid carbon dioxide is known as dry ice. (1983 - 1 Mark)
(vii) Anhydrous HCl is a bad conductor of electricity but aqueous HCl is a good conductor; (1985 - 1 Mark)
(viii) Graphite is used as a solid lubricant; (1985 - 1 Mark)
(ix) Fluorine cann ot be prepared from fluorides by chemical oxidation. (1985 - 1 Mark)
(x) The mixture of hydrazine and hydrogen peroxide with a copper(II) catalyst is used as a rocket propellant. (1987 - 1 Mark)
(xi) Orthophosphorus acid is not tribasic acid. (1987 - 1 Mark)
(xii) The molecule of magnesium chloride is linear whereas that of stannous chloride is angular. (1987 - 1 Mark)
(xiii) Valency of oxygen is generally two whereas sulphur shows valency of two, four and six. (1988 - 1 Mark)
(xiv) H3PO3 is a dibasic acid. (1989 - 1 Mark)
(xv) Phosphine has lower boiling point than ammonia. (1989 - 1 Mark)
(xvi) Ammonium chloride is acidic in liquid ammonia solvent. (1991 - 1 Mark)
(xvii) The hydroxides of aluminium and iron are insoluble in water. However, NaOH is used to separate one from the other. (1991 - 1 Mark)
(xviii) Bond dissociation energy of F2 is less than that of Cl2. (1992 - 1 Mark)
(xix) Sulphur dioxide is a more powerful reducing agent in an alkaline medium than in acidic medium. (1992 - 1 Mark)
(xx) The experimentally determined N – F bond length in NF3 is greater than the sum of the single covalent bond radii of N and F. (1995 - 2 Marks)
(xxi) Mg3N2 when reacted with water gives off NH3 but HCl is not obtained from MgCl2 on reaction with water at room temperature. (1995 - 2 Marks)
(xxii) (SiH3)3N is a weaker base than (CH3)3N. (1995 - 2 Marks)
Solution.
(i) Carbon exists in various allotropic forms like diamond, graphite, coal, etc. Diamond consists of a threedimensional structure of sp3 hybridised carbon atoms bonded through very strong covalent bonds. It makes it hard and useful as an abrasive.
Graphite, on the other hand, is made up of a two dimensional sheet like structure made of sp2 hybridised carbon atoms. These layers of carbon atoms are held together by relatively weak van der Waal’s forces and can, therefore, slip over one another imparting lubricating properties to graphite.
(ii) Sulphur consists of S8 rings held together by weak van der Waal’s forces. As sulphur melts at 119ºC, these van der Waal’s forces are overcome and S8 rings slip and roll over one another giving rise to a clear mobile liquid. Above 160ºC, the S8 rings begin to open up and form long chains which gets tangled with each other, thereby gradually increasing the viscosity.
(iii) NOTE : HI cannot be prepared by heating hydrogen iodide with conc. H2SO4 because it is a strong reducing agent and reduces H2SO4 to SO2 and is itself oxidised to iodine.
H2SO4 + 2HI → SO2 + I2 + 2H2O
Hence HI is prepared by heating iodides with conc. phosphoric acid.
3KI + H3PO4 → K3PO4 + 3HI
(iv) In H3PO4 and H3PO3 the P atom is attached to 3 and 2 OH groups respectively. The H atom of these P – OH bonds are ionisable. This clearly shows that H3PO4 is tribasic and H3PO3 is dibasic.
(v) Liquor ammonia possesses high vapour pressure at room temperature and thus before opening a bottle of liquor ammonia, it should be cooled to lower the pressure of NH3 inside the bottle, otherwise NH3 will bump out of the bottle.
(vi) Solid CO2 is technically known as dry ice because it sumblimes without leaving any stain on surface.
(vii) Anhydrous HCl, being a non - polarcovalent compound, is a bad conductor however an aqueous solution of HCl is ionised (Fajan’s rule) to give H+ and Cl– ions and is a good conductor.
(viii) In graphite, out of four valence electrons, only three form covalent bonds (sp2 hybridisation) with three other carbon atoms. This forms hexagonal rings as sheets of one atom thickness. These sheets are held together by weak attractive forces. One electron of each carbon atom is free and this enables these thin sheets to slide over one another. For this reason graphite is a soft material with lubricating properties.
(ix) The standard reduction potential of fluorine is highest and thus it cannot be oxidized by any reagent
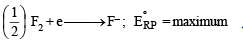
(x) The mixture of N2H4 and H2O2 (in presence of Cu (II) catalyst) is used as a rocket propellant because the reaction is highly exothermic and large volume of gases are evolved, which can propel a rocket.
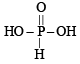
(xi) Orthophosphorus acid is a dibasic acid a s it h as 2–OH groups in its formula :
(xii) In MgCl2, Mg is sp hybridised while in SnCl2, Sn is sp2 hybridised (hence the molecule is angular).
(xiii) NOTE : Oxygen is the 2nd most electronegative element after the fluorine and thus invariably show negative oxidation state.
Further more, it has 2s22p4 configuration and thus requires only two electrons to complete its octet to show –2 oxidation state. Although sulphur also possess ns2np4 configuration but due to availability of d-orbitals in their outer most shell –2, +2, +4, +6 oxidation state are also shown. Oxygen, however, shows only –2 oxidation state due to non-availability of d-orbitals in its outermost shell.
(xiv) NOTE : H3PO3 is a dibasic acid because it contains two OH groups in its molecule.
In the two P–OH bonds, the hydrogen is ionisable. [For structure see part (xi)]
(xv) NOTE : As compared to P, N atom has higher electronegativity and small size and shows H-bonding.
Thus ammonia molecule show association where as phosphine does not.
(xvi) It is due to self ionization of NH3, the reaction is
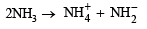
Thus on addition of NH4Cl the concentration of NH+4 radical increases and therefore NH4Cl acts as an acid in liquid NH3.
(xvii) In excess of NaOH the h ydroxide of Al becomes soluble due to the formation of meta-aluminate.
(xviii) The repulsive forces between fluorine atoms are high due to its small size and high electronegativity. It makes dissociation of F – F bond easy. So bond dissociation energy of F2 is less than Cl2
(xix) The reducing nature of SO2 is represented as
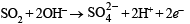
Hence with the increase of OH– (alkalinity) the forward reaction is favoured.
(xx) Nitrogen and fluor ine both are small and have h igh electron density, they repel the bonded pair of electrons leading to larger bond length than expected.
(xxi)N3– being smaller in size and high charge present on it make it more susceptible to hydrolysis :
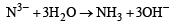
Cl– being a weak conjugate base does not undergo hydrolysis. MgCl2 is stronger electrolyte and so it is not hydrolysed
(xxii) In (SiH3)3N, lone pair of electr ons on nitrogen is involved in pπ – dπ back bonding, while in (CH3)3N no such pπ – dπ back bondin g is possible because of absence of d orbitals in carbon so (CH3)3N is more basic than (SiH3)3N.
Q.7. State with balanced equations what happens when :
(i) White phosphorous (P4) is boiled with a strong solution of sodium hydroxide in an inert atmosphere. (1982/87 - 1 Mark)
(ii) Sodiumiodate is treated with sodium bisulphite solution. (1982 - 1 Mark)
(iii) Dilute nitric acid is slowly reacted with metallictin. (1987 - 1 Mark)
(iv) Potassium permanganate is reacted with warm solution of oxalic acid in the presence of sulphuric acid. (1987 - 1 Mark)
(v) Iodate ion reacts with bisulphite ion to liberate iodine. (1988 - 1 Mark)
(vi) Phosphorus reacts with nitric acid to give equimolar ratio of nitric oxide and nitrogen dioxide. (1988 - 1 Mark)
(vii) Hypophosphorous acid is heated. (1989 - 1 Mark)
(viii) Sodium bromate reacts with fluorine in presence of alkali. (1989 - 1 Mark)
(ix) Sodium chlorate reacts with sulphur dioxide in dilute sulphuric acid medium. (1989 - 1 Mark)
(x) Write balanced equations for the preparation of crystalline silicon from SiCl4. (1990 - 1 Mark)
(xi) Write balanced equations for the preparation of phosphine from CaO and white phosphorus. (1990 - 2 Marks)
(xii) Write balanced equations for the preparation of ammonium sulphate from gypsum, ammonia and carbon dioxide. (1990 - 1 Mark)
(xiii) Aqueous solution of sodiumnitrate is heated with zinc dust and caustic soda solution. (1990 - 1 Mark)
(xiv) Sodium iodate is added to a solution of sodium bisulphite. (1990 - 1 Marks)
(xv) Sodium nitrite is produced by absorbing the oxides of nitrogen in aqueous solution of washing soda. (1991 - 1 Mark)
(xvi) Nitrogen is obtained in the reaction of aqueous ammonia with potassium permanganate. (1991 - 1 Mark)
(xvii) Elemental phosphorus reacts with conc. HNO3 to give phosphoric acid. (1991 - 1 Mark)
(xviii) Sulphur is precipitated in the reaction of hydrogen sulphide with sodium bisulphite solution. (1991 - 1 Mark)
(xix) Phosphorus is treated with concentrated nitric acid. (1997 - 1 Mark)
OR
Manufacture of phosphoric acid from phosphorus. (1997 - 1 Mark)
(xx) Reaction of aluminium with aqueous sodium hydroxide. (1997 - 1 Mark)
(xxi) Aluminium sulph ide gives a foul odour wh en it becomes damp. Write a balanced chemical equation for the reaction. (1997 - 2 Marks)
(xxii) P4O10+ PCl5 → (1998 - 1 Mark)
(xxiii) SnCl4 + C2H5Cl + Na → (1998 - 1 Mark)
Solution. (i) Phosphine gas (PH3) is evolved when white phosphorous is boiled with aqueous NaOH or alcoholic solution of potassium hydroxide.
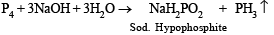
(ii) This is a method used to prepare I2.
5NaHSO3 + 2NaIO3 → 3NaHSO4 + 2Na2SO4 + I2 + H2O
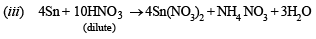
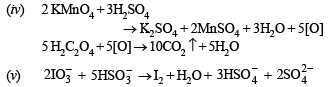
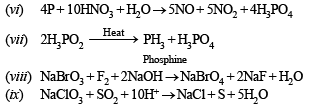
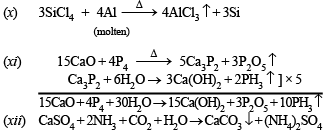
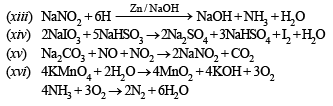
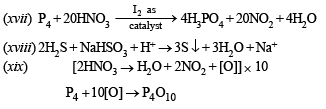
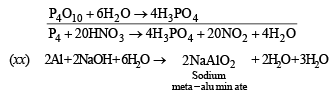
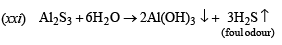
Foul odour, on damping of Al2S3 is due to formation of H2S gas, which smells like rotten eggs.
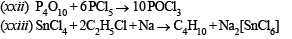
Q.8. Show with equation s h ow the following compound is prepared (equations need not be balanced) sodium thiosulphate from sodium sulphite. (1982 - 1 Mark)
Solution. By boiling Na2SO3 solution with powder of sulphur in absence of air sodium thiosulphate is prepared. Unreacted S is removed, filtrate is evaporated to give crystals of sod. thiosulphate.
Na2SO3 + S → Na2S2O3
Q.9. Give balanced equations for the extraction of aluminium from bauxite by electrolysis. (1982 - 2 Marks)
Solution. Extraction of aluminium from bauxite :
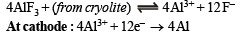
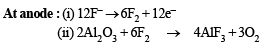
Q.10. State the conditions under which the following preparation is carried out. Give the necessary equations which need not be balanced : Alumina from aluminium. (1983 - 1 Mark)
Solution.
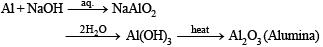
Q.11. Write down the resonance structures of nitrous oxide. (1985 - 2 Marks)
OR
Write the two resonance structures of N2O that satisfy the octet rule. (1990 - 1 Mark)
Solution. N2O has two principal resonance structures :
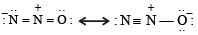
Q.12. Write down the balanced equations for the reactions when:
(i) a mixture of potassium ch lor ate, oxalic acid and sulphuric acid is heated; (1985 - 1 Mark)
(ii) ammonium sulphate is heated with a mixture of nitric oxide and nitrogen dioxide. (1985 - 1 Mark)
Solution. (i) KClO3 + 2H2C2O4 + H2SO4 → KHSO4 + HCl + 6CO2 + 3H2O
(ii) (NH4)2SO4 + NO + NO2 → 2N2 + 3H2O + H2SO4
Q.13. What happens when :
(i) hydrogen sulphide is bubled through an aqueous solution of sulphur dioxide. (1985 - 1 Mark)
(ii) tin is treated with concentrated nitric acid. (1985 - 1 Mark)
(iii) Pb3O4 is treated with nitric acid. (1985 - 1 Mark)
Solution. (i) H2S oxidises into S,
SO2 + 2H2S → 3S + 2H2O

(iii) Pb3O4 + 4HNO3 → 2Pb(NO3)2 + 2H2O + PbO2↓
Q.14. Arrange the following in :
(i) increasing bond strength (1986 - 1 Mark)
HCl, HBr, HF, HI
(ii) HOCl, HOClO2, HOClO3, HOClO in increasing order of thermal stability. (1988 - 1 Mark)
(iii) CO2, N2O5, SiO2, SO3 in the order of increasing acidic character. (1988 - 1 Mark)
(iv) Increasing order of extent of hydrolysis :
CCl4, MgCl2, AlCl3, PCl5, SiCl4 (1991 - 1 Mark)
Ans. (i) HI < HBr <HCl < HF,
(ii) HOCl < HOClO < HOClO2 < HOClO3
(iii) SiO2 < CO2 < N2O5 < SO3,
(iv) CCl4 <MgCl2 < AlCl3 < SiCl4 < PCl5
Solution.
(i) HI < HBr < HCl < HF
The strength of H–X bond decreases from HF to HI.
The larger is H–X bond length, lower is the bond energy, lesser is the bond strength.
(ii) HOCl < HOClO < HOClO2 < HOClO3 As the number of oxygen atoms increase, the -ve charge dispersal becomes more and more from Cl atom due to more electronegativity of oxygen atom and thus lesser is the charge on Cl atom, more will be its stability.
(iii) SiO2 < CO2 < N2O5 < SO3.
Among oxides of the non-metals, the acidic strength increases with oxidation state. Hence SO3 (O.S. of S = +6) is most acidic followed by N2O5 (O.S. of N = +5) and CO2 and SiO2 (O.S. of C and Si = +4). Further CO2 is more acidic than SiO2 because of small size of C-atom.
(iv) Since carbon has no d-orbital, it cannot extend its coordination number beyond four, its halides are not attacked (hydrolysed) by water. On the other hand, silicon have vacant d-orbitals to which water molecules can coordinate and hence their halides are hydrolysed by water.
NOTE : Increasing order of extent of hydrolysis
CCl4 < MgCl2 < AlCl3 < SiCl4 < PCl5
Q.15. Mention the products formed in the following :
(i) Chlorine gas is bubbled through a solution of ferrous bromide. (1986 - 1 Mark)
(ii) Iodine is added to a solution of stannous chloride. (1986 - 1 Mark)
(iii) Sulphur dioxide gas, water vapour and air are passed over heated sodium chloride. (1986 - 1 Mark)
Solution.
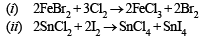
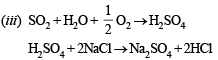
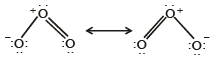
Q.16. Write the two resonance structures of ozone which satisfy the octet rule. (1991 - 1 Mark)
Solution. The two resonating structures of ozone are :
Q.17.
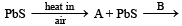 Pb + SO2; Identify A and B. (1991 - 2 Marks)
Pb + SO2; Identify A and B. (1991 - 2 Marks)
Ans. [A]-PbO, [B]-heat in the absence of air
Solution.
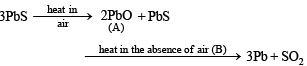
Q.18. Complete and balance the following chemical reactions :
(i) Red phosphorus is reacted with iodine in presence of water. (1992 - 1 Mark)
P + I2 + H2O → ........... + ........... .
(ii) Anhydrous potassium nitrate is heated with excess of metallic potassium. (1992 - 1 Mark)
KNO3(s) + K(s) → ........... + ........... .
(iii) NH3 + NaOCl → ........ + ........ (1993 - 1 Mark)
(iv) Sn + 2KOH + 4H2O → ........ + ......
Solution.(i) 2P + 3I2 + 6H2O → 2H3PO4 + 6HI
(ii) 2KNO3 + 10K → 6K2O + N2
Q.19. Draw the structure of P4O10 and identify the number of single and double P—O bonds. (1996 - 3 Marks)
Ans. 12, 4
Solution.
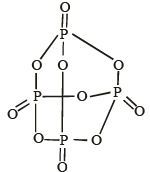
Number of P – O single bonds = 12
Number of P – O double bonds = 4
Q.20. Gradual addition of KI solution to Bi(NO3)3 solution initially produces a dark brown precipitate which dissolves in excess of KI to give a clear yellow solution. Write chemical equations for the above reactions. (1996 - 2 Marks)
Solution.At first Bi(NO3)3 hydrolyses to give nitric acid which, being an oxidising agent, oxidises potassium iodide liberating free iodine responsible for dark brown precipitate. Iodine dissolves in excess of potassium iodide forming soluble KI3 imparting yellow colour to solution
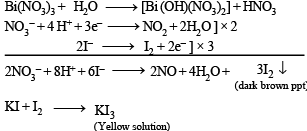
Q.21. Complete the following chemical equations :
(a) KI + Cl2 →
(b) KCIO3 + l2 →
Justify the formation of the products in the above reactions. (1996 - 2 Marks)
Solution. 2KI + Cl2 → 2KCl + I2
Since Cl2 is more powerful oxidising agent than I2, Cl2 is able to displace I– to form I2.
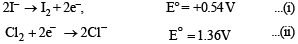
On subtracting eq. (i) from eq. (ii), we get
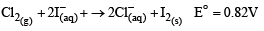
(b) 2KClO3 + I2 → 2KIO3 + Cl2 .
Here 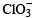 is more powerful oxidising agent than
is more powerful oxidising agent than
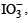 so Cl is displaced by I.
so Cl is displaced by I.
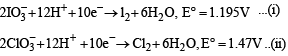
On subtracting eq. (i) from eq. (ii), we get
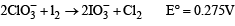
|
446 docs|929 tests
|





















