Short & Long Answer Questions: Polymers | Chemistry for JEE Main & Advanced PDF Download
Q.1 Give examples of semi synthetic polymers.
Answer: Cellulose acetate (rayon) and cellulose nitrate.
Q.2 Which type of polymer nylon-6.6, is ?
Answer: Polymide polymer or (condensation polymer).
Q.3 Give example of thermoplastic.
Answer: Polythene, polystyrene and polyvinyl.
Q.4 Give the name of monomer ofnylon-2, nylon-6.
Answer: Glycine and amino caproic acid.
Q.5 What is the significance of number 6,6 and 6 in nylon-6,6 and nylon-6?
Answer: In monomer of nylon-6,6 (hexamethylene demine and atopic acid) 6,6 refers to number of carbon atoms. Both have six carbon atoms. In nylon-6, monomer is caprolactum which has also six carbon atoms.
Q.6 What is novolac?
Answer: The initial product formed when phenol and formaldehyde are polymerised is novolac.
Q.7 What is bakelite?
Answer: Bakelite is a thermosetting polymer of phenol and formaldehyde.
Q.8 Which type of polyesters are biodegradable?
Answer: Aliphatic polyesters.
Q.9 Name the polymer used in the insulation of electrical wire.
Answer: Styrene-Butadiene copolymer. (SBR or Buna-S)
Q.10 Name the polymer used in making non-stick kitchen wares.
Answer: Teflon.
Q.11 Name a polymer used as a substitute for wool.
Answer: PAN (Poly acrylonitrile) or orlon.
Q.12 Name a polymer used in making unbreakable crockery.
Answer: Melamine-formaldehyde co-polymer.
Q.13 Name the polymer used in making C.D.
Answer: Polystyrene and polycarbonates.
Q.14 Name a polymer used in the manufacture of paints and lacquers.
Answer: Glyptal.
Q.15 Name the monomer of (a) PHBV and (b) Nylon-2, Nylon-6.
Answer: (a) 3-Hydroxy butanoic acid and 3-Hydroxy pentanoic acid. (b) Glycine and amino caproic acid.
Q.16 Name the catalyst used in free radical addition polymerization as initiator.
Answer: Benzoyl peroxide.
Q.17 Name the catalyst used in the preparation of high density polythene.
Answer: Triethyl aluminium and titanium tetrachloride (Zieeler Natta catalyst).
Q.18 Name the catalyst used in the preparation of low density polythene.
Answer: Traces of dioxygen or peroxide initiator.
Q.19 What is difference between novolac and bakelite?
Answer: The initial linear product formed by the polymerisation of phenol and formaldehyde is called novolac. It is soft. Whereas novolac on heating with formaldehyde undergoes cross linking to form bakelite. It is hard.
Q.20 What percentage of sulphur is used to obtain tyre rubber from natural rubber?
Answer: 5% sulphur is used to obtain tyre rubber.
Q.21 What is the role of sulphur in the vulcanisation of rubber?
Answer: On vulcanisation, sulphur forms cross links at the reactive site of double bonds and thus rubber gets stiffened.
Q.22 Give one use of novolac.
Answer: Used in paints.
Q.23 Give chemical name of orlon.
Answer: Poly acrylonitrile (PAN).
Q.24 Name the polymer used for making handle of pressure cooker.
Answer: Bakelite.
Q.25 What is the role of benzoyl peroxide in the polymerization of ethene?
Answer: Produces free radical which initiates the chain reaction.
Q.26 Name the polymer of phthalic acid and ethylene ?
Answer: Glyptal.
Q.27 What are macromolecules?
Answer: Long chain organic molecules which have very high molecular mass are often called macromolecules.
Q.28 Define polymerization.
Answer: The process of formation of a polymer from respective monomers is called polymerization.
Q.29 What are polymers?
Answer: Polymer is defined as very large molecule having high molecular mass formed by repetition of some small monomer units.
Q.30 How are polymers classified on the basis of source?
Answer: On the basis of source polymers are classified as : (i) Natural polymers : Found in plants and animals e.g. Proteins, cellulose, starch, etc. (ii) Semi synthetic: Cellulose derivatives. e.g. Cellulose acetate (rayon) and cellulose nitrate. (iii) Synthetic : e.g. Plastic, nylon-6,6, Buna-S.
Q.31 How are polymers classified on the basis of structure.
Answer: On the basis of structure they are classified as: (i) Linear polymers : e.g. polythene, polyvinyl chloride. (ii) Branched chain : e.g. low density polythene. (iii) Cross linked or network polymers : e.g. Bakelite Melamine.
Q.32 Define copolymer. Give example.
Answer: The polymers made by polymerisation of two different molecules are termed as copolymers. e.g. Buna-S, Buna-N, etc.
Q.33 What are elastomers?
Answer: Elastomers are the polymers in which polymer chains are held by weakest intermolecular force. e.g. Buna-S, Buna-N.
Q.34 Define fibres.
Answer: Fibres are the thread forming solids which possess high tensile strength and high modulus. They are held together by strong intermolecular forces such as H-bond. e.g. nylon 6,6, terylene.
Q.35 What are thermoplastics?
Answer: Thermoplastics are polymers which can be remoulded again and again. e.g. polythene, polystyrene, etc.
Q.36 What are thermosetting polymers?
Answer: Thermosetting polymers are those which cannot be remoulded again. e.g. Bakelite, urea-formaldehyde resins, etc.
Q.37 Give difference between thermoplastic and thermosetting plastics.
Answer:
| Thermoplastic | Thermosetting | |
| 1. | These are linear and slightly branched long chain molecule | These polymers are cross linked and heavily branched molecules. |
| 2. | These can be remoulded again and again. | These are not remoulded again. |
Q.38 How is low density polythene prepared?
Answer: Low density polythene is prepared by heating ethene under high pressure of 1000 to 2000 atmosphere at a temperature 350 to 570 K in the presence of traces of dioxide and peroxide initiator.
Q.39 How is high density polythene prepared?
Answer: High density polythene is formed when addition polymerisation of ethene takes place in hydrocarbon solvent in the presence of catalyst such as triethyl aluminium and titanium tetrachloride (also called Ziegler Natta catalyst) at a temperature 333 K to 343 K and under a pressure 6-7 atmosphere.
Q.40 What is vulcanisation of rubber?
Answer: The process of heating a mixture of raw rubber with sulphur at temperature range between 373 K to 415 K in order to make it stiffened is called vulcanization of rubber.
Q.41 What are biodegradable polymers?
Answer: Polymers which do not persist in environment and decompose after some time due to bacterial action are known as biodegradable polymers. e.g. PHBV, nylon-2-nylon-6.
Q.42 What are the bad effects of non-biodegradable polymers?
Answer: Non biodegradable polymers are quite resistant to the environmental degradation processes and cause accumulation of solid polymeric waste materials. These waste materials remain underground for quite a long time and cause acute environmental problems.
Q.43 What are biopolymers?
Answer: Polymers present in plants and animals are called biopolymers. Biomolecules such as carbohydrate, protein, etc. are biopolymers.
Q.44 How does vulcanisation improve the quality of rubber?
Answer: Natural rubber becomes soft at high temperature (> 335 K) and brittle at low temperature (< 283 K). So, in order to make it hard it is vulcanised by adding sulphur. Sulphur forms cross links at the reactive sites of double bonds and thus rubber gets stiffened.
Q.45 How will you differentiate between low density and high density polythene?
Answer:
| Low density polythene | High density polythene | |
| 1. | It is prepared under high pressure of 1000-2000 atm. | It is prepared under low pressure of 6-7 atm. |
| 2. | It is tough but flexible. | It is tougher and harder. |
| 3. | Highly branched structure. So, it has low density due to less closely packed. | It is linearly arranged and has a high density due to close packing. |
Q.46 Give the uses of low density and high density polythene.
Answer: Low density polythene is used in insulation of electricity carrying wires and manufacture of squeeze bottles, toys and flexible pipes. High density polythene is used in manufacture of bucket dustbin bottles, etc.
Q.47 What is synthetic rubber? Give examples.
Answer: Synthetic rubber is any vulcanisable rubber like polymer which is capable of getting stretched to twice its length. e.g. Neoprene, Buna-N, Buna-S, etc.
Q.48 What are polyamide polymers? Give example.
Answer: Those polymers in which amide (-CONH-) linkage is present in the chain are called polyamides. e.g. nylon 6,6, nylon-6.
Q.49 What are polyester polymers? Give example.
Answer: Polyester polymers are the poly condensation products of dicarboxylic acids and diols. e.g. dacron (terylene).
Q.50 Classify the following as thermoplastic and thermosetting polymers. Bakelite, urea-formaldehyde resin, polythene, polystyrene, polyvinyl chloride.
Answer: (i) Bakelite - Thermosetting polymer (ii) Urea formaldehyde resins- Thermosetting polymer. (iii) Polyethene - Thermoplastic polymers (iv) Polystyrene - Thermoplastic polymers. (v) polyvinyl chloride - Thermoplastic polymers.
Q.51 Classify the following as elastomers and fibres nylon-6,6, terylene, buna-S, buna-N.
Answer: (i) Nylon 6,6- Fibre (ii) Terylene - Fibre (iii) Buna-N- Elastomer (iv) Buna-S- Elastomer
Q.52 Arrange the following polymers in order of increasing intermolecular force. Bakelite, Nylon-6,6, Polythene, Neoprene.
Answer: Neoprene < Polythene < Nylon 6,6 < Bakelite.
Q.53 How polymers are different from macromolecules? Explain with example.
Answer: A polymer always consists of large number of repeating structural units but a macromolecule may or may not consist of repeating structural units. e.g. Proteins and nucleic acids should be regarded as macromolecules but not polymer since their molecules do not contain repeating structural units where as cellulose is a polymer because it contains P-D-glucose as repeating units.
Q.54 Why always purest monomer is used in free radical polymerization?
Answer: If impurity is present then alkene monomers may act as chain transfer agents or chain inhibitors. Therefore, the monomer in free radical polymerization is taken as pure.
Q.55 Which property is used to determine the molecular mass of polymers?
Answer: Physical and chemical properties are used to determine the molecular mass of polymers.
Q.56 Which type of polymerization gives a polymer containing carbon atoms only in the main chain?
Answer: Addition polymerization gives a polymer containing carbon atoms only in the main chain.
Q.57 Which type of polymerization gives a polymer containing hetero atoms?
Answer: Condensation polymerization gives a polymer containing hetero atoms.
Q.58 Name a substance which inhibits free radical polymerization.
Answer: Benzoquinone inhibits free radical polymerization. For structure and details, consult section 16.2.
Q.59 To which class of polymers does Nylon-6,6 belong?
Answer: It is a condensation polymer and is polymide in nature.
Q.60 What is mode of free radical polymerisation in alkenes?
Answer: It is addition polymerisation. For details, consult section 16.2.
Q.61 Write the structures of the monomers used for getting the following polymers? (a) PVC (b) Teflon (c) PMMA
Answer: (a)CH2=CHCl (b) CF2=CF2 (c) 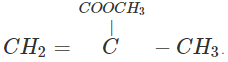
Q.62 Why should one always use purest monomer in free radical polymerisation?
Answer: Impurities of other substances if present, may inhibit or hinder the chain propagation. They can also act as chain transfer agents and inhibit or even terminate the original chain reaction.
Q.63 Why does styrene undergo anionic polymerisation easily?
Answer: In styrene, an electron withdrawing phenyl group is present. As a result, the nucleophile or anionic attack can easily take place leading to anionic polymerisation.
Q.64 Why is cationic polymerisation preferred in case of vinylic monomers containing electron donating groups?
Answer: Electron donating groups tend to increase the electron density on the monomer unit. As a result, cationic attack can easily take place. For example, cationic polymerisation in propene with an electron releasing CH3 group with+I effect.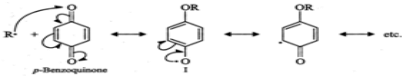
Q.65 Depict a free radical mode of addition polymerisation in isoprene.
Answer: Free radical polymerisation in isoprene is initiated by a free radical attack.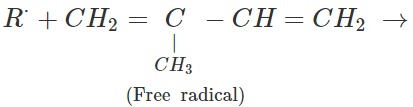
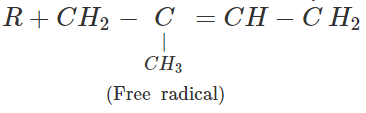
Free radicals participate in further polymerisation.
Q.66 Why are the numbers 6,6 and 6 put in the names of nylon 6,6 and nylon 6?
Answer: These numbers indicate the number of carbon atoms in the monomer units involved in the two varieties of nylon. nylon 6,6 : Adipic acid (six 'C' atoms) and hexamethylene diamine (six 'C' atoms) nylon 6 : ε-Aminocaproic acid.
Q.67 Polymers are always macromolecules but macromolecules are not always polymers. Explain.
Answer: In polymers, monomers which participate may be either same (homo polymers) or different (co-polymers). These are all macromolecules in nature. Biopolymers such as starch, proteins and nucleic acids are also macromolecules. But some macromolecules like chlorophyll, haemoglobin etc. do not have any monomer units and are not polymers.
Q.68 Can a co-polymer be formed both in addition and condensation polymerisation?
Answer: Yes it can be formed in both the cases. For example, Buna-S is a copolymer of styrene, 1, 3-butadiene and sodium and is an addition polymer. Nylon-6,6 is a condensation co-polymer in which monomers are different. i.e., adiptic acid and hexamethylene diamine.
Q.69 Explain how does 1, 3-butadiene polymerise by different routes.
Answer: Butadiene is a conjugated diene and its free radical polymerisation can occur in two ways: (i) When the polymerisation takes place at C1 and C4 of butadiene, an unbranched polymer results. It can exist either as trans-polybutadiene or as cis isomer or as the mixture of both.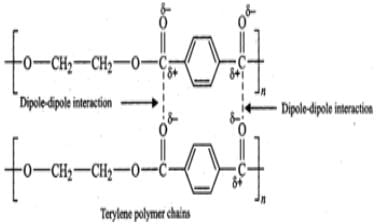
(ii) 1, 3 butadiene can also undergo polymerisation at C1 and C2 to give polyvinyl polyethene as the product.
Q.70 Are polyesters and polyacrylates same? Justify your answer.
Answer: Polyesters and polyacrylates are different types of polymers and differ in the following characteristics. (i) Polyacrylates are homo polymers while the polyesters are co-polymers in nature. (ii) The mode of synthesis of polyacrylates is addition polymerisation while that of polyesters is condensation polymerization. (iii) Polymerization occurs across C=C bond in polyacrylates whereas in polyesters it is through ester linkage.
Q.71 Write the structures of the monomers of the following polymers :
(a) 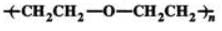 (b)
(b) 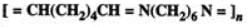
Answer: and
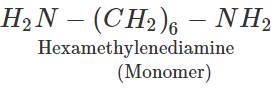
Q.72 Draw the repeating structural units of the step-growth polymers you would expect to obtain from the following reactions.
BrCH2CH2CH2Br+HOCH2CH2CH2OH 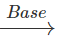 HOCH2CH2OH+HOOC(CH2)6COOH
HOCH2CH2OH+HOOC(CH2)6COOH 
Answer: (a) −CH2CH2CH2O− (b) −OCH2CH2O−CO(CH2)6CO−
Q.73 (a)Is 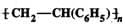 a homo polymer or a copolymer? (b) Is it an addition or condensation polymer?
a homo polymer or a copolymer? (b) Is it an addition or condensation polymer?
Answer: (a) The repeating structural unit of the given polymer contains only one type of monomer unit, i.e. styrene, C6H5CH=CH2, therefore, it is a homo polymer. (b) Since the monomer contains a double bond, and the polymer does not, therefore, it is an addition polymer.
Q.74 (a) Can a copolymer be formed in both addition and condensation polymerization? Explain. (b) Can a homo polymer be formed in both addition and condensation polymerization? Explain.
Answer: (a) Yes, copolymers can be formed both in addition and condensation, polymerization. For example, Buna- S is an addition copolymer of styrene and 1, 3-butadiene while nylon-6, 6, bakelite and polyester are condensation copolymers. (b) Yes, homo polymers can be formed both in addition and condensation polymerization. For example, polythene, PVC, PMMA, PAN, neoprene, etc. are examples of addition homo polymers while nylon-6 is an example of condensation homo polymer.
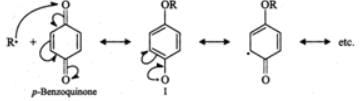
Q.75 How does the presence of benzoquinone inhibit the free radical polymerization of a vinyl derivative?
Answer: Benzoquinone reacts with radical of the growing polymer chain (R⋅) to form a new radical (I) which is extremely unreactive since it is highly stabilized by resonance. Because of the lack of reactivity of this new radical further growth of the polymer chain is interrupted and hence the reaction stops.
Q.76 Differentiate the following pairs of polymers based on the property mentioned against each. (i) Novolac and bakelite (structure). (ii) Buna-S and terylene (intermolecular forces of attraction).
Answer: (i) Novolac is a linear but bakelite is a cross- linked polymer of phenol and formaldehyde. For structures refer to pages 15/14-15/15.
(ii) Terylene contains ester functional groups which are polar in nature. Therefore, the intermolecular forces of attraction involved in terylene are strong dipole-dipole interactions as shown below :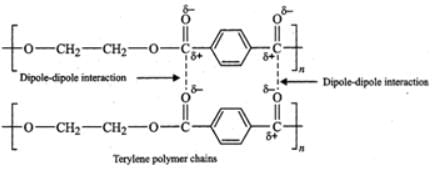
Buna-S, on the other hand, does not have polar functional groups. It has only non-polar hydrocarbon chains and hence has only weak Van der Waals forces of attraction as shown below :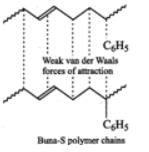
Q.77 State the significance of numbers 6 and 6, 6 in the polymer names nylon-6 and nylon-6, 6. or In nylon 6, 6 what does the designation 6, 6 mean.
Answer: Nylon-6 means that it is a condensation polymer of only one type of monomer molecules containing six carbon atoms, i.e., caprolactam. Nylon-6, 6, on the other hand, implies that it is a condensation polymer of two types of monomer molecules each containing six carbon atoms, i.e., adipic acid(HOOCCH2CH2CH2CH2COOH) and hexamethylenediamine (H2NCH2CH2CH2CH2CH2CH2NH2).
Q.78 What is the repeating unit in the condensation polymer obtained by combining HO2CCH2CH2COOH (succinic acid) and H2NCH2CH2NH2 (ethylenediamine).
Answer: The repeating structural unit is obtained by condensing at both ends of both the bifunctional molecules, i.e., Succinic acid (HOOCCH2CH2COOH) and ethylenediamine with the elimination of water molecules, i.e., −OCCH2CH2CO−NHCH2CH2NH-.
|
334 videos|651 docs|300 tests
|
FAQs on Short & Long Answer Questions: Polymers - Chemistry for JEE Main & Advanced
| 1. What are polymers and why are they important in NEET? |  |
| 2. How are polymers formed? |  |
| 3. What are the different types of polymers? |  |
| 4. How do polymers contribute to environmental concerns? |  |
| 5. What are the applications of polymers in medicine? |  |





















