Class 8 Science Chapter 4 Question Answers - Combustion and Flame
Q25: Explain global warming and its causes.
Ans: Global warming is the increase in the average temperature of Earth's atmosphere and oceans, reason for the same is:
1. Increasing population that leads to increased use of fossil fuels and agriculture, fossil fuels releases carbon dioxide gas which causes global warming.
2. Because of increased population more number of people releases carbon dioxide gas during respiration process and that contributes to global warming.
3. Increased demand of agriculture for our increasing population is also one of the reason of global warming as manures used in agriculture contains methane gas.
Q26: What are the effects of global warming?
Ans: Global warming is the increase in the average temperature of Earth's atmosphere and oceans, it results in melting of the glacier of the polar region which leads to rise in sea level, causing flood in coastal areas. Even low lying coastal region may get permanently submerged under water because of global warming.
Q27: Explain why it is difficult to burn a heap of green leaves but we can easily burn dry leaves?
Ans: A heap of green leaves contains lot of water and has very high ignition temperature, as water is a natural fire extinguisher it do not allow leaves to catch fire easily where as dry leaves contains no water and have low ignition temperature thus they can catch fire easily.
Q28: In an experiment 3.5 kg of a fuel was completely burnt. The heat produced was measures to be 160,000 kj. Calculate the calorific value of the fuel.
Ans: Calorific value of the fuel = Amount of heat energy produced / weight of fuel burnt
= 160000/3.5 kj/kg
= 45714.28 kj/kg
Q29: What is fuel? Give some examples of a fuel.
Ans. The combustible substances which produce heat and sometimes light on combustion is called fuel. Fuel may be solid, liquid and gas. Wood, charcoal, petrol and kerosene are some of the examples of fuel.
Q30: What are combustible and non-combustible substances? Explain with examples.
Ans. The substances in which combustion takes place are called combustible substances. For example: wood, paper, coal. The substances in which no combustion takes place are called non‑combustible substances. For example: Glass, iron nail.
Q31: Why does a matchstick not burn of its own?
Ans. The ignition temperature for the burning of matchstick is more than room temperature. So it does not catch fire on its own. When the stick is rubbed then due to friction it gets its ignition temperature and starts to burn.
Q32: Can you burn a piece of wood by bringing a lighted matchstick near it?
Ans. The piece of wood cannot burn by bringing a lighted matchstick near it. It is because the heat produced by matchstick is not sufficient to attain the ignition temperature of wood. So we use paper or kerosene oil to start fire in wood piece.
Q33: We can boil water in a paper cup while paper catches fire easily. Explain the process.
Ans. We take two paper cups. Take some water in one cup and keep the other empty. Heat both the cups. Empty cup starts to burn but the cup containing water does not burn. If we continue heating the water in the cup it starts boiling. The heat supplied to the paper cup is transferred to water by conduction. So in the presence of water the ignition temperature of paper is not reached. Hence, it does not burn.
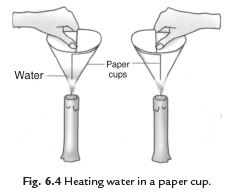
Q34: How does fire brigade works to extinguish fire?
Ans. When fire brigade arrives, it pours water on the fire. Water cools the combustible material, so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapour also surrounds the combustible material, helping in the cutting off the supply of air. So the fire is extinguished.

Q35: What is the job of a fire extinguisher?
Ans. There are three requirements for producing fire: fuel, air, proper temperature. Fire can be controlled by removing one or more of these requirements.
The job of a fire extinguisher is to cut off the supply of air or to bring down the temperature of the fuel or both. In most of the cases fuel cannot be eliminated.
Q36: Explain the structure of a flame.
Ans. There are following three parts of a flame:
(i) Outer zone: It is non-luminous and the hottest zone.
(ii) Middle zone: It is less hot and yellow coloured zone.
(iii) Inner zone: It is dark zone and least hot part.
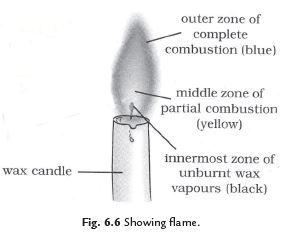
Q37: Why is the colour of outer zone is blue while middle zone is yellow coloured?
Ans. Outermost zone: It is blue coloured part because complete combustion takes place in this part due to sufficient amount of oxygen. It is the hottest part of the flame.
Middle Zone: The colour of the middle zone is yellow because incomplete combustion takes place in this part for the lack of oxygen. It is less hot part than outer part of the flame.
Q38: What is calorific value of a fuel?
Ans. The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its calorific value. The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg).
Q39: What is acid rain? Write its effects.
Ans. The oxides of sulphur and nitrogen dissolve in rain water to form acids.
Such rain containing acids is called acid rain. It is very harmful for crops, buildings and soil.
|
91 videos|296 docs|44 tests
|
FAQs on Class 8 Science Chapter 4 Question Answers - Combustion and Flame
| 1. What is combustion? |  |
| 2. How does combustion produce heat and light? |  |
| 3. What are the different types of flames? |  |
| 4. How is a fire extinguished? |  |
| 5. Why is it important to control combustion? |  |






















