Faraday's Laws of Electrolysis | Chemistry for JEE Main & Advanced PDF Download
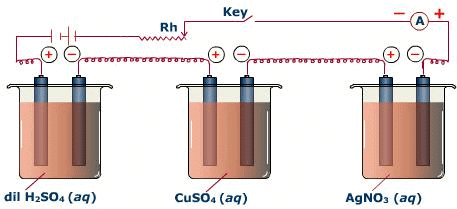 Fig: Electrolytic cellElectrolytic cell: The electrochemical cell which facilitates a chemical reaction through the induction of electrical energy is known as an electrolytic cell. This process of carrying out non-spontaneous reactions under the influence of electric energy is termed as electrolysis.
Fig: Electrolytic cellElectrolytic cell: The electrochemical cell which facilitates a chemical reaction through the induction of electrical energy is known as an electrolytic cell. This process of carrying out non-spontaneous reactions under the influence of electric energy is termed as electrolysis.
Michael Faraday conducted an extensive investigation on electrolysis of solutions and melts of electrolytes. He was the first scientist who described the quantitative aspects of Laws of Electrolysis. He proposed two laws to explain the quantitative aspects of electrolysis popularly known as Faraday’s laws of electrolysis namely first law of electrolysis and the second law of electrolysis.
First Law of Electrolysis:
It is one of the primary laws of electrolysis. It states, during electrolysis, the amount of chemical reaction which occurs at any electrode under the influence of electrical energy is proportional to the quantity of electricity passed through the electrolyte.
Second Law of Electrolysis:
During electrolysis, when the same quantity of electricity passes through the electrolytic solution, a number of different substances liberated are proportional to their chemical equivalent weights (Equivalent weight is defined as the ratio of the atomic mass of metal and the number of electrons required for reducing the cation).
From these laws of electrolysis, we can deduce that the amount of electricity needed for oxidation- reduction depends on the stoichiometry of the electrode reaction. For example:
Na++e− → Na
As we can observe, one mole of the electron is required for the reduction of one mole of sodium ions. We know that charge on one electron is equal to 1.6021 × 10–19C. Therefore, the charge on one mole of electrons is equal to:
NA × 1.6021 × 10–19C = 6.02 × 1023mol–1 × 1.6021 × 10–19C = 96487 C mol–1
This quantity of electricity is defined as one Faraday and is denoted by F. Hence; one Faraday is defined as the charge carried per unit mole of electrons.
The product of an electrolytic reaction depends on the nature of the material being electrolyzed and the type of electrodes used.
In the case of an inert electrode such as platinum or gold, electrode does not participate in the chemical reaction and acts only as a source or sink for electrons. While, in the case of a reactive electrode, electrode participates in the reaction.
Hence, different products are obtained for electrolysis in the case of reactive and inert electrodes. Oxidizing and reducing species present in the electrolytic cell and their standard electrode potential too, affect the products of electrolysis.
Factors Affecting Products of Electrolysis:
1. Products of electrolysis depend on the material being electrolyzed. In other words, the nature of electrolyte governs the process of electrolysis. The process is fast for a strong electrolyte whereas for weak electrolyte an extra potential better known as over potential is required.
Products of electrolysis depend on upon the value of this over potential too.
2. Products of electrolysis depend on the nature of electrodes too. That is, in the case of the inert electrode (say gold, platinum), it doesn’t participate in the reaction whereas if the electrode used is reactive in nature it takes part in the reaction.
3. Various oxidising and reducing species present in the electrolytic cell do affect the products of electrolysis.
4. The products of electrolysis depend on standard electrode potentials of the different oxidizing and reducing species present in the electrolytic cell.
5. In case of multiple reactions, product of electrolysis depends on the standard electrode potential of various reactions taking place. For example, electrolysis of aqueous solution of sodium chloride. Out of the multiple reduction reactions taking place, the reduction reaction which has highest value of standard electrode potential takes place at cathode. Similarly, out of the multiple oxidation reactions, the oxidation reaction which has the lowest value of standard electrode potential takes place at anode.
|
334 videos|656 docs|300 tests
|
FAQs on Faraday's Laws of Electrolysis - Chemistry for JEE Main & Advanced
| 1. What are Faraday's laws of electrolysis? |  |
| 2. What is the first law of electrolysis proposed by Faraday? |  |
| 3. How does Faraday's second law of electrolysis explain the relationship between different substances in electrolysis? |  |
| 4. Can Faraday's laws be applied to any electrolyte? |  |
| 5. How are Faraday's laws used in practical applications? |  |

















