Intermolecular Forces: Definition and Types | General Awareness - Bank Exams PDF Download
| Table of contents |

|
| What are Intermolecular Forces in Chemistry? |

|
| Intramolecular vs Intermolecular Forces |

|
| Types of Intermolecular Forces |

|
| Which Type of Intermolecular Force Is the Strongest? |

|
What are Intermolecular Forces in Chemistry?
Intermolecular forces, often abbreviated to IMF, are the attractive and repulsive forces that arise between the molecules of a substance. These forces mediate the interactions between individual molecules of a substance.
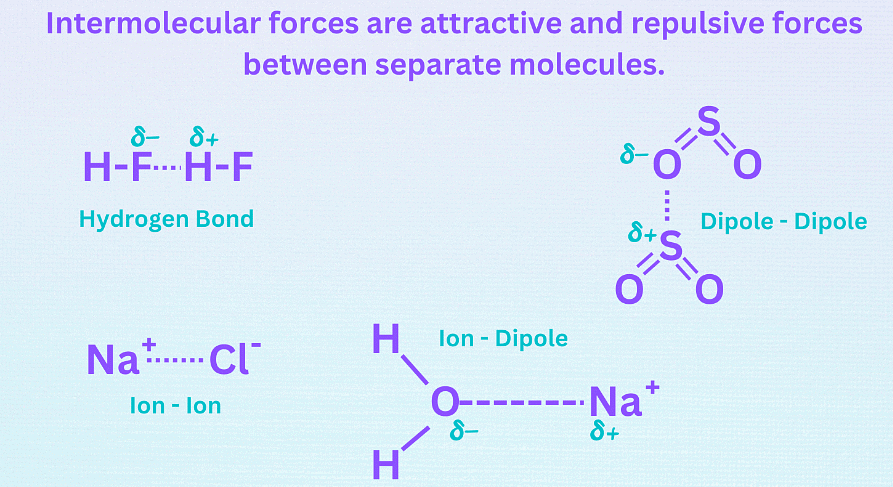 Intermolecular Forces
Intermolecular Forces
- Intermolecular forces are attractive and repulsive forces between atoms, groups of atoms, or ions in separate molecules.
- The three main types of intermolecular forces are:
Hydrogen bonding (dipole-dipole forces),
Ion-dipole forces (and ion-induced dipole forces),
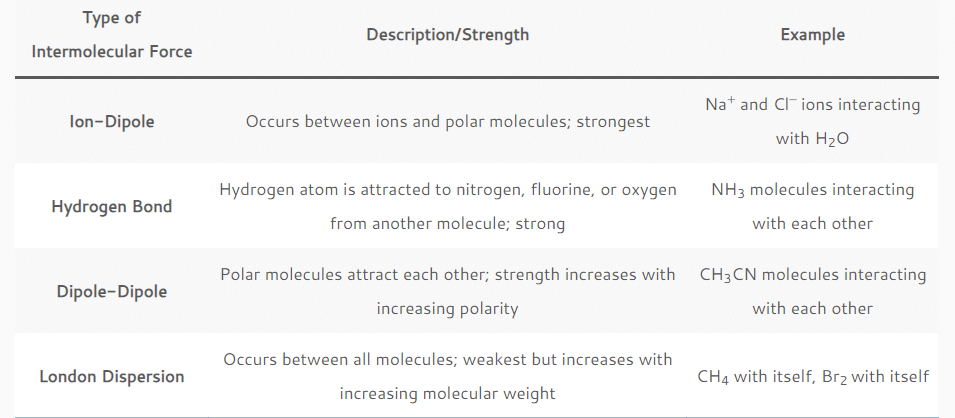
and Van der Waals forces (Debye force, London dispersion force, Keesom force). - Ion-dipole forces are the strongest intermolecular forces, followed by hydrogen bonding, other dipole-dipole forces, and dispersion forces. Van der Waals forces are the weakest intermolecular forces.
Intramolecular vs Intermolecular Forces
Intermolecular forces pertain to interactions between separate molecules, whereas intramolecular forces refer to the attractive and repulsive forces within individual molecules, governing chemical bonds and molecular structure.
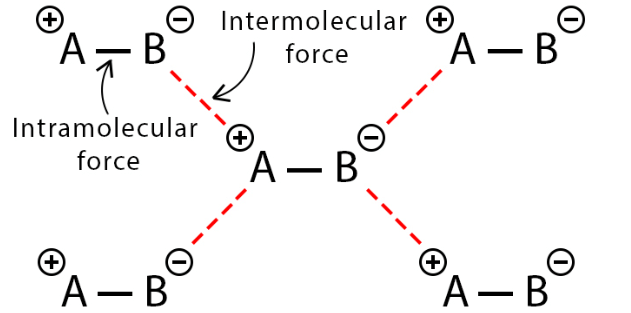 Intermolecular and Intramolecular Forces
Intermolecular and Intramolecular Forces
- In both instances, these forces operate between atoms or groups of atoms. It's worth noting that intermolecular forces are relatively weaker when compared to intramolecular forces, yet both types are instrumental in shaping molecules, influencing their properties, and governing their interactions.
- In visual representations, intermolecular forces are often depicted as dashed lines, while intramolecular forces (bonds) are represented as solid lines.
The table below compares and contrasts inter and intramolecular forces.
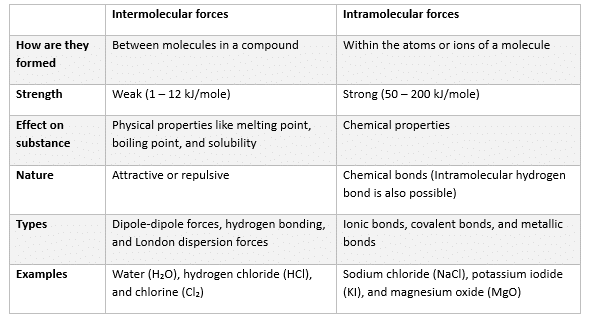
Types of Intermolecular Forces
Intermolecular forces can either attract (opposite electrical charges) or repel (like charges), but the main classes of intermolecular forces deal with attraction. The main types of intermolecular forces are:
- Ion-dipole forces
- Ion-induced dipole forces
- Dipole-dipole forces (including hydrogen bonding)
- Induced dipole forces
- Dipole-induced-dipole forces
- Hydrogen Bonding
1. Ion-dipole forces
As the name suggests, this type of intermolecular force exists between an ion and a dipole (polar) molecule. You will remember that an ion is a charged atom, and this will be attracted to one of the charged ends of the polar molecule.
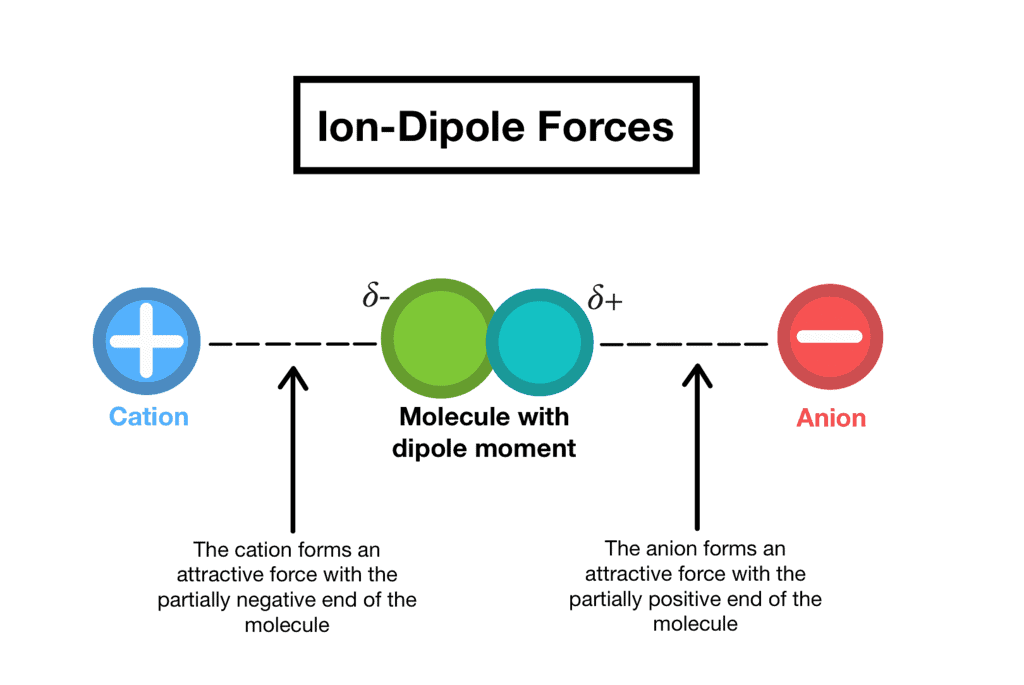
- A positive ion will be attracted to the negative pole of the polar molecule, while a negative ion will be attracted to the positive pole of the polar molecule. This can be seen when sodium chloride (NaCl) dissolves in water.
- The positive sodium ion (Na+) will be attracted to the slightly negative oxygen atoms in the water molecule, while the negative chloride ion (Cl−) is attracted to the slightly positive hydrogen atoms. These intermolecular forces weaken the ionic bonds between the sodium and chloride ions so that the sodium chloride dissolves in the water.
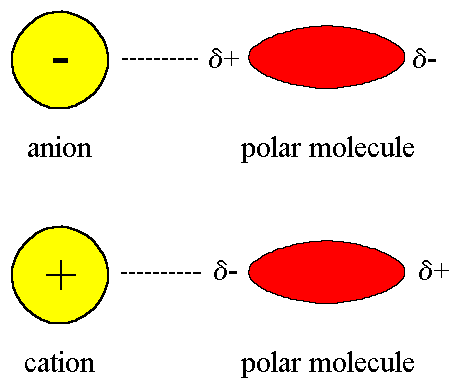 Ion- dipole forces
Ion- dipole forces
- This is a simplified diagram to highlight the regions of positive and negative charge. When sodium chloride dissolves in water it can more accurately be shown as:
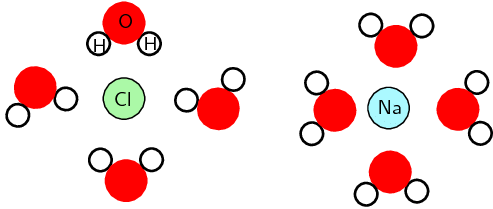 Region of positive and negative charge when NaCl is dissolved in water
Region of positive and negative charge when NaCl is dissolved in water
2. Ion-induced-dipole forces
Similar to ion-dipole forces these forces exist between ions and non-polar molecules.
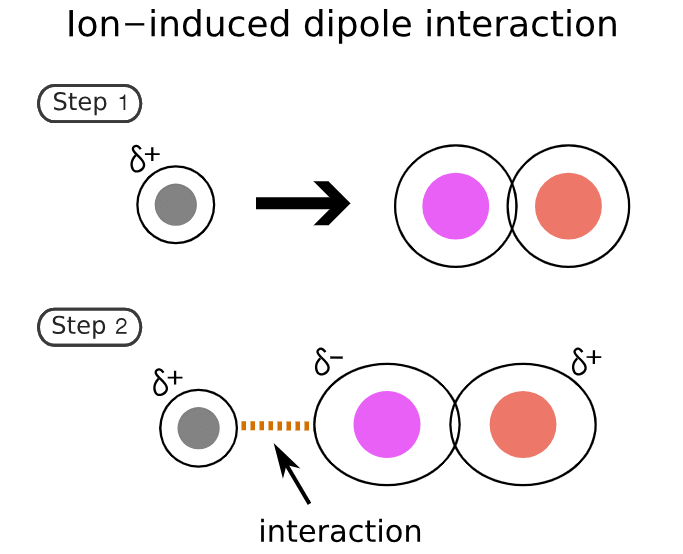
- The ion induces a dipole in the non-polar molecule leading to a weak, short-lived force that holds the compounds together.
- These forces are found in hemoglobin (the molecule that carries oxygen around your body). Hemoglobin has Fe2+ ions. Oxygen (O2) is attracted to these ions by ion-induced dipole forces.
3. Dipole-dipole forces
When one dipole molecule comes into contact with another dipole molecule, the positive pole of the one molecule will be attracted to the negative pole of the other, and the molecules will be held together in this way.
- Examples of materials/substances that are held together by dipole-dipole forces are HCl, SO2 and CH3Cl.
- One special case of this is hydrogen bonding.
 Dipole- dipole interactions
Dipole- dipole interactions
4. Induced dipole forces
These intermolecular forces are also sometimes called “London forces” or “momentary dipole” forces or “dispersion” forces.
- We know that while carbon dioxide is a non-polar molecule, we can still freeze it (and we can also freeze all other non-polar substances). This tells us that there must be some kind of attractive force in these kinds of molecules (molecules can only be solids or liquids if there are attractive forces pulling them together). This force is known as an induced dipole force.
- In non-polar molecules, the electronic charge is usually evenly distributed but it is possible that at a particular moment in time, the electrons might not be evenly distributed (remember that the electrons are always moving in their orbitals). The molecule will have a temporary dipole.
- In other words, each end of the molecules has a slight charge, either positive or negative. When this happens, molecules that are next to each other attract each other very weakly. These forces are found in the halogens (e.g. F2 and I2) and in other non-polar molecules such as carbon dioxide and carbon tetrachloride.
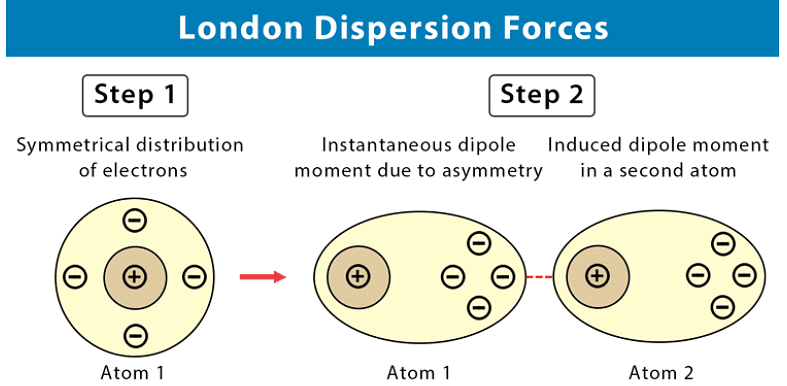
- All covalent molecules have induced dipole forces. For non-polar covalent molecules these forces are the only intermolecular forces. For polar covalent molecules, dipole-dipole forces are found in addition to the induced dipole forces.
When the noble gases condense, the intermolecular forces that hold the liquid together are induced dipole forces.
5. Dipole-induced-dipole forces
- This type of force occurs when a molecule with a dipole induces a dipole in a non-polar molecule. It is similar to an ion-induced dipole force. An example of this type of force is chloroform (CHCl3) in carbon tetrachloride (CCl4).
The following image shows the types of intermolecular forces and the kinds of compounds that lead to those forces.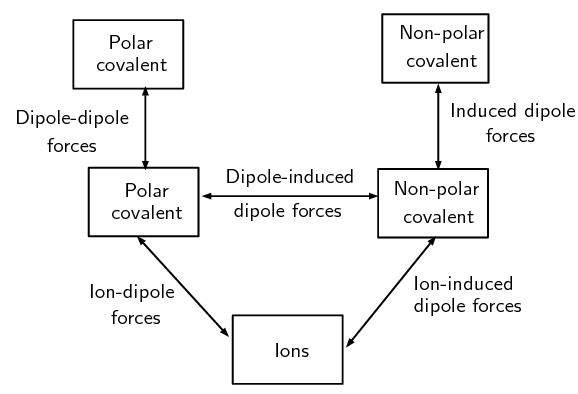 The types of intermolecular forces. The boxes represent the type of compound while the lines represent the type of force
The types of intermolecular forces. The boxes represent the type of compound while the lines represent the type of force - The last three forces (dipole-dipole forces, dipole-induced dipole forces, and induced dipole forces) are sometimes collectively known as van der Waals' forces. We will now look at a special case of dipole-dipole forces (i.e. Hydrongen Bonding) in more detail.
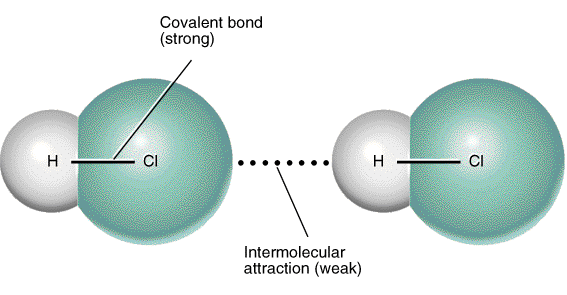
6. Hydrogen bonds
- As the name implies, this type of intermolecular bond involves a hydrogen atom. When a molecule contains a hydrogen atom covalently bonded to a highly electronegative atom (O, N, or F) this type of intermolecular force can occur. The highly electronegative atom on one molecule attracts the hydrogen atom on a nearby molecule.
- Water molecules, for example, are held together by hydrogen bonds between the hydrogen atom of one molecule and the oxygen atom of another.
- Hydrogen bonds are a relatively strong intermolecular force and are stronger than other dipole-dipole forces. It is important to note, however, that hydrogen bonds are weaker than the covalent and ionic bonds that exist between atoms
Do not confuse hydrogen bonds with actual chemical bonds. Hydrogen bonding is an example of a case where a scientist named something believing it to be one thing when in fact it was another. In this case the strength of the hydrogen bonds misled scientists into thinking this was actually a chemical bond, when it is really just an intermolecular force.
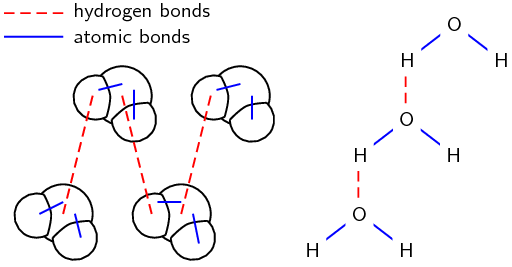 Two representations showing the hydrogen bonds between water molecules: space-filling model and structural formula Solved Example:
Two representations showing the hydrogen bonds between water molecules: space-filling model and structural formula Solved Example:
Question: Which intermolecular forces are found in:
- Hydrogen fluoride (HF)
Hydrogen fluoride is a polar covalent molecule. (It is linear and not symmetrical.) So the type of intermolecular force is dipole-dipole forces.
- Methane (CH4)
Methane is a non-polar covalent molecule. (It is tetrahedral and symmetrical.) So the type of intermolecular force is induced dipole forces.
- Potassium chloride in ammonia (KCl in NH3)
Potassium chloride is an ionic compound. Ammonia is a polar covalent molecule. (It is trigonal pyramidal and not symmetrical.) So the type of intermolecular force is ion-dipole forces.
- Krypton (Kr)
Krypton is a noble gas. So the type of intermolecular force is induced dipole forces.
Which Type of Intermolecular Force Is the Strongest?
The nature of the chemical species involved in intermolecular forces matters, so there is no hard-and-fast ranking of strongest to weakest intermolecular forces. But, ion-dipole interactions tend to be the strongest, followed by hydrogen bonding, other types of dipole-dipole bonding, and London dispersion forces.
|
365 videos|700 docs|149 tests
|
FAQs on Intermolecular Forces: Definition and Types - General Awareness - Bank Exams
| 1. What are intermolecular forces in chemistry? |  |
| 2. What is the difference between intramolecular and intermolecular forces? |  |
| 3. What are the types of intermolecular forces? |  |
| 4. How do intermolecular forces affect the physical properties of substances? |  |
| 5. What are some examples of substances that exhibit intermolecular forces? |  |
















