Class 10 Science: CBSE Sample Question Paper (2019-20) - 4 | Science Class 10 PDF Download
Class-X
Science-086
SAMPLE QUESTION PAPER 2019-20
TIME: 3 Hrs.
M.M: 80
General Instructions:
1. The question paper comprises three sections - A, B and C. Attempt all the sections.
2. All questions are compulsory.
3. Internal choice is given in each section.
4. All questions in Section A are one - mark questions comprising MCQ, VSA type and
assertion-reason type questions. They are to be answered in one word or in one sentence.
5. All questions in Section B are three - mark, short - answer type questions. These are to be answered in about 50 - 60 words each.
6. All questions in Section C are five - mark, long - answer type questions. These are to be answered in about 80 - 90 words each.
7. This question paper consists of a total of 30 questions.
Section A
Q.1. A lake has been polluted by sewage. On comparison with the sample of unpolluted water, the water in the lake is found to have increased content of some components. Identify these components. (1 Mark)
Ans: Sewage is rich in organic substances. The decomposition of these substances by decomposers increases nitrogenous compounds in water bodies polluted by sewage.
Q.2. While applying Fleming’s right-hand rule the central (middle) finger of right hand indicates (1 Mark)
(a) the direction of magnetic field.
(b) the direction of rotation of conductor.
(c) the direction of current being flown.
(d) the direction of induced current.
Ans: (d) As per Fleming’s right-hand rule the central finger of right-hand points in the direction of induced current, when forefinger is along the direction of magnetic field and thumb points along the direction of motion of the conductor.
Q.3. Answer question numbers 3(a)-3(d) on the basis of your understanding of. the following paragraph and the related studied concepts.
In ancient times, wood was the most common source of heat energy. The energy of flowing water and wind was also used for limited activities. The exploitation of coal as a source of energy made the industrial revolution possible. Increasing industrialization has led to a better quality of life all over the world. It has also caused the global demand for energy to grow at a tremendous rate. The growing demand for energy was largely met by the fossil fuels - coal and petroleum. Our technologies were also developed for using these energy sources. However, these fuels were formed over millions of years ago and there are only limited reserves. The fossil fuels are non-renewable sources of energy, so we need to conserve them. If we were to continue consuming these sources at such alarming rates, we would soon run out of energy! In order to avoid this, alternate sources of energy were explored.
But we continue to be largely dependent on fossil fuels for most of our energy requirements. Burning fossil fuels has other disadvantages too. Burning of these fuels cause about the air pollution.
The oxides of carbon, nitrogen and-sulphur that are released on burning fossil fuels are acidic oxides. These lead to acid rain, which affects our water and soil resources. In addition to the problem of air pollution, we also encounter the greenhouse effect of gases like carbon dioxide.
(a) What was the most common fuel in ancient times? (1 Mark)
(b) Which fossil fuel is responsible for the industrial revolution? (1 Mark)
(c) What type of fuel is fossil fuel? (1 Mark)
(d) Oxides of which elements are released on the burning of fossil fuel? (1 Mark)
Ans:
(a) Wood.
(b) Coal.
(c) Non-renewable.
(d) Oxides of carbon, nitrogen and Sulphur.
Q.4. Understand the given flow chart and answer the following questions.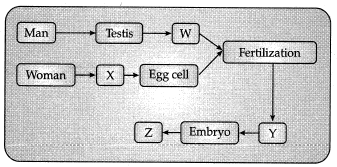
(a) Which of the following represents W, X, Y and Z? (1 Mark)
(b) Where the process of fertilization does Lakes place in female body? (1 Mark)
(c) What is the main function of part X? (1 Mark)
(d) What is menstruation? (1 Mark)
Ans: (a) (ii) The label W- Sperm, X- Ovary, Y- Zygote, Z- Foetus
(b) Fertilization takes place in fallopian tube.
(c) Produces egg or female gamete, female sex hormone/ estrogen.
(d) It is the periodic breakdown of uterine lining and its removal along with blood and mucous in (post pubertal stage of a) human female.
Q.5. Which component is being reduced in the given reaction ? (1 Mark)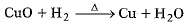
(a) H2O
(b) Cu
(c) H2
(d) CuO
Ans: (d) CuO is getting reduced to metallic Cu by loosing its oxygen.
Q.6. Slaked lime has the formula (1 Mark)
(a) CaO
(b) Ca(OH)2
(c) CaO.Ca(OH)2
(d) CaOCl2
Ans: Ca(OH)2
Q.7. In an electrical circuit three incandescent bulbs A, B and C of rating 40 W, 60 W and 100 W respectively are connected in parallel to an electric source. Which of the following is likely to happen regarding their brightness ? (1 Mark)
(a) Brightness of all the bulbs will be the same
(b) Brightness of bulb A will be the maximum
(c) Brightness of bulb B will be more than that of A
(d) Brightness of bulb C will be less than that of B
Ans: (c)
Q.8. According to the evolutionary theory, formation of a new species is generally due to : (1 Mark)
(a) sudden creation by nature.
(b) accumulation of variations over several generations.
(c) clones formed during asexual reproduction.
(d) movement of individuals from one habitat to another.
Ans: (b) According to the evolutionary theory, formation of a new species is generally due to accumulation of variations over several generations.
Q.9. The genotype of the height of an organism is written as Tt. What conclusion may be drawn? (1 Mark)
(a) There is one allele for height with two different forms
(b) There are at least two different alleles for the gene for height
(c) The allele for height has at least two different genes
(d) There are two different genes for height, each having a single allele
Or
During fertilisation, the zygote received Y-chromosome from the father. The sex of this particular zygote will be
(a) girl
(b) boy
(c) girl or a boy
(d) None of these
Ans: (b) The genotype of the height of an organism is written as Tt. There are at least two different alleles for the gene for height. One allele is dominant and is represented by capital letter, i.e. T and another allele is recessive and is represented by small letter, i.e. t.
Or
(b) If a Y-chromosome carrying sperm fertilises with an ovum (XX), the zygote will develop into a male (boy) child.
Q.10. Select the incorrect statement. (1 Mark)
(а) Tendril of a pea plant and phylloclade of Opuntia are analogus.
(b) Sweet potato and potato are analogous.
(c) Wing of a bird and wing of a bat are analogous.
(d) Radish and Ginger are analogous.
Ans: (a) They are homologous not analogous.
Q.11. Asexual reproduction takes place through budding in: (1 Mark)
(a) Amoeba
(b) Yeast
(c) Plasmodium
(d) Leishmania.
Ans: (b)
Q.12. Rays from Sun converge at a point 15 cm in front of a concave mirror. Where should an object be placed so that size of its image is equal to the size of the object ? (1 Mark)
(a) 15 cm in front of the mirror
(b) 30 cm in front of the mirror
(c) Between 15 cm and 30 cm
(d) More than 30 cm in front of the mirror, in front of the mirror
Ans: (b) The rays from Sun i.e., from infinity, are parallel to principal axis after reflection converge at a point, known as focus. Therefore, focal length (F) of concave mirror is 15 cm. Since, the same size, real and inverted image is formed by concave mirror when object is placed at focus 2 F or centre of curvature. So to form same size image, object will be place at 15 x 2 = 30 cm.
Q.13. In each of the following questions, a statement of Assertion is given by the corresponding statement of Reason. Of the statements, mark the co rrect answer as (1 Mark)
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are true, but Reason is not the correct explanation of Assertion.
(c) If Assertion is true, but Reason is false.
(d) If Assertion is false, but Reason is true.
Assertion: Fertilisation is a unique feature in flowers.
Reason: It is followed by pollination.
Ans: (c) Fertilisation is a unique feature of flowers because the sperm released by a pollen is involved in fertilisation.
The sperm unites with the egg and this forms a zygote. It is followed by embryo formation and not pollination.
Q.14. The ability of carbon to form bonds with other carbon atoms is called catenation. (1 Mark)
Ans: True.
Section B
Q.15. Describe the events that occur after the fertilisation of germ cells in plants. (3 Mark)
Or
Explain the following methods of contraception by giving one example of each.
(i) Barrier method
(ii) Chemical method
(iii) Surgical method
Ans: The fusion of the germ cells (or fertilisation) forms zygote. The events that occur after fertilisation in plants are
(i) After fertilisation, the zygote divides several times to form an embryo within the ovule.
(ii) The ovule develops a tough coat and is gradually converted into a seed.
(iii) The ovary grows rapidly and ripens to form a fruit.
(iv) The sepals, petals, stamens and style may fall off.
Or
(i) Barrier method It involves the usage of certain products or devices which prevent the meeting of gametes and help in birth control, e.g. condom, diaphragm, intrauterine Contraceptive Device (IUCD).
(ii) Chemical method It involves usage of chemicals called spermicides, which are applied in vagina in order to kill sperms. It can only be used with condoms or diaphragm.
(iii) Surgical method Birth control from this method involves vasectomy and tubectomy. In vasectomy, a small portion of the vas deferens is cut and tied to prevent sperm transfer. In tubectomy, a small portion of oviduct is cut and tied thus, blocking the path of egg. The disadvantage is that both the processes are irreversible.
Q.16. A coil of insulated copper wire is connected to a galvanometer. What would happen if a strong bar magnet is
(a) pushed into the coil ?
(b) withdrawn from inside the coil ?
(c) held stationary inside the coil ?
Give justification for each observation. (3 Mark)
Ans: (a) When a strong bar magnet is pushed into the coil, the magnetic field inside the coil increases and so an induced current is produced due to phenomenon of electromagnetic induction. As a result, the galvanometer gives a deflection.
(b) When the magnet is withdrawn from inside the coil, magnetic field of coil decreases and an induced current is produced in the opposite direction. So, galvanometer again shows a deflection but it is in the opposite direction.
(c) When magnet is held stationary inside the coil, magnetic field of the coil remains unchanged and there is no induced current. Hence, galvanometer does not show any deflection.
Q.17. An element 'X ' has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of 'X Also write group number, period number and valency of 'X'. (3 Mark)
Ans: Atomic number of X
= Mass number of X - No. of neutrons
= 35 - 18 = 17
Therefore, Electronic configuration of X
= 2 , 8 , 7
Group number = 17
Period no. =3
Valency = 8 - 7 = 1
Q.18. What is feedback mechanism of hormonic regulation. Take the example of insulin to explain this phenomenon.
OR
Nervous and hormonal systems together perform the function of control and coordination in human beings. Justify this statement with the help of an example. (3 Mark)
Ans: Mechanism by which the amount of any chemical increases or decreases resulting in secretion of the related hormone.
Example: When sugar level rises, insulin secretion increases. When sugar level falls, insulin secretion reduces.
OR
For nervous and hormonal systems to control and coordinate in human beings, hypothalamus plays an important role in receiving the neural / nerve signals from brain and release hormones.
E.g. In situation of iodine deficiency, hypothalamus releases hormones to stimulate pituitary gland, it further sends stimulating hormone to thyroid gland to secrete thyroxine that regulates carbohydrate metabolism.
Q.19. Why did Mendel choose pea plants for his experiments? (3 Mark)
Ans: Due to the following reasons, Mendel choose pea plant for his experiments
(i) Pea plants were self-pollinating.
(ii) They were easy to cultivate and had a short lifespan.
(iii) They showed clearly defined and contrasting traits such as seed colour (yellow-green), height (tall-dwarf), etc.
Q.20. (a) Write the function of the following in the human alimentary canal:
(i) Saliva
(ii) HCl in stomach
(iii) Bile juice
(iv) Villi
(b) Write one function each of the following enzymes :
(i) Pepsin (ii) Lipase (3 Mark)
Ans: (a) Functions of the following :
(i) Saliva - (1) It helps in lubrication of food.
(2) It contains the enzyme salivary amylase which breaks down complex starch to disaccharide sugar i.e., maltose.
(ii) HCl in stomach - (1) It makes the medium acidic.
(2) It kills bacteria.
(3) It activates precursor of enzyme i.e., pepsinogen - pepsin.
(iii) Bile juice - (1) It makes the medium alkaline.
(2) It helps in emulsification of fats so that they can be acted upon by the enzymes and digested.
(iv) Villi — (1) They are present in small intestine.
(2) They increase the surface area for absorption of digested food.
(b) Function of enzymes :
(i) Pepsin - Helps in digestion of proteins in stomach.
(ii) Lipase - Helps in digestion of fats.
Q.21. What is the Junction of receptors in our body ?
Think of situations where receptors do not work properly. What problems are likely to arise ? (3 Mark)
Ans: All information from environment is detected by receptors. Receptors pass information in the form of electrical impulses to brain by nerve cells and brain send information to effector organs for response. When receptors do not work properly, information from environment (stimuli) cannot be detected and our body cannot respond accordingly.
Q.22. Name, state and explain with an example the rule used to determine the direction of force experienced by a current carrying conductor placed in a uniform magnetic field. (3 Mark)
Ans: Fleming's Left Hand Rule : The direction of force which acts on the current carrying conductor placed in a magnetic field is given by Fleming's left hand rule. It states that if the forefinger, thumb and middle finger of left hand are stretched mutually perpendicular and the forefinger point along the direction of external magnetic field, middle finger indicates the direction of current, then thumb points along the direction of force acting on the conductor.
Example : When an electron enters a magnetic field at right angles, the direction of force on electron is perpendicular to the direction of magnetic field and current according to this rule.
Q.23. Acid are the chemical substances which have a sour taste, whereas bases are the chemical substances which are bitter in taste, soapy to touch.
(a) Give a chemical test to distinguish between an acid or a base.
(b) Choose strong acid, strong base and weak acid from the following compounds:
H2CO3, HNO3, CH3COOH, NaOH,NH4OH, KOH, Ca(OH)2,HCl. (3 Mark)
Ans: (a) Acids change the colour of blue litmus to red while bases change the colour of red litmus to blue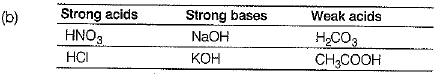
Q.24. What is biodiversity ? Why are forests considered as “biodiversity hotspots” ?
List two factors responsible for causing deforestation. (3 Mark)
Ans: Biodiversity is the existence of variety of plants and animal species of an area. Forests are considered as ‘biodiversity hotspots’ as they are very rich in the availability of various life forms as they provide necessary resources for various life forms. Deforestation is caused due to :
(i) Expanding population and hence need for more housing and other resources.
(ii) Industrialisation and growth,
(iii) Overgrazing by cattle.
(iv) Cutting down of trees by different stakeholders etc.
Section C
Q.25. Differentiate between metals and non-metals on the basis of their chemical properties.
OR
A man went door to door posing as a goldsmith.
He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set o f gold bangles to him which he dipped in a particular solution. The bangles sparkled like a new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used ? (5 Mark)
Ans:
OR
The man used Aqua regia which is a freshly prepared mixture of cone. HCl and cone. HNO3 in the ratio of 3 : 1. This mixture can dissolve gold but either of these acids cannot dissolve, alone. Aqua regia is a highly corrosive, fuming' liquid and is one of the few reagents that can dissolve gold and platinum.
Q.26. (a) What was the basis of Mendeleev's classification of elements ?
(b) List two achievements of Mendeleev's Periodic table.
(c) List any two observations which posed a challenge to Mendeleev's periodic law.
OR
Explain giving justification the trends in the following properties of elements, on moving from left to right in a period, in the Modern Periodic Table :
(a) Variation of valency.
(b) Change of atomic radius.
(c) Metallic to non-metallic character.
(d) Electronegative character.
(e) Nature of oxides. (5 Mark)
Ans: (a) Atomic mass
(b) (i) He could classify all the 63 elements known at that time.
(ii) He left gaps for the yet to be discovered elements.
(iii) He predicted the properties of such element.
(c) (i) Position of isotopes
(ii) Irregular increase in atomic masses in going from one element to the next, making the prediction of undiscovered elements difficult.
(iii) Position of Hydrogen.
Q.27. (i) Name the metal for each case:
(a) It does not react with cold as well as hot water but reacts with steam.
(b) It does not react with any physical state of water.
(ii) What is a thermite reaction. Give its equation and state one use of this reaction? (5 Mark)
Ans: (i) (a) Aluminium
(b) Copper
(ii) The reaction between iron (III) oxide (Fe2O3) and aluminium produces large amount of heat, i.e. exothermic reaction. This reaction is called the-thermite reaction.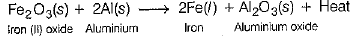
This reaction is used to join railway tracks or cracked machine parts.
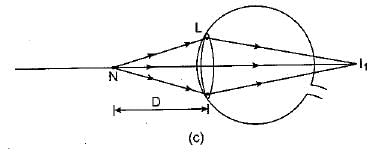
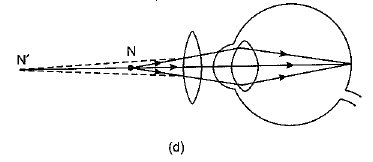
Q.28. A person is unable to see objects distinctly placed within 50 cm from his eyes.
(а) Name the defect of vision the person is suffering from and list its two possible causes.
(b) Draw a ray diagram to show the defect in the above case.
(c) Mention the type of lens used by him for the correction of the defect and calculate its power. Assume that the near point for the normal eye is 25 cm.
(d) Draw a labelled diagram for the correction of the defect in the above case. (5 Mark)
Ans: (a) The man is suffering from hypermetropia or long sightedness defect.
Two possible causes of hypermetropia are :
(i) Either the power of the eye lens is less (or focal length of eye lens is too long) due to less curvature of cornea, or
(ii) Shortening of eyeball due to some genetic defect.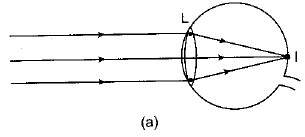
Q.29. (a) Define resistance of a conductor. State the factors on which resistance of a conductor depends. Name the device, which is often used to change the resistance without. changing the voltage source in an electric circuit.
(b) Calculate the resistance of 50 m length of wire of cross sectional area 0.01 square mm and of resistivity 5 x 10-8 Ωm. (5 Mark)
Ans:(a) It is defined as the opposition offered to the flow of current by a conductor. It depends upon:
(i) Nature of the material of the conductor.
(ii) Length of the conductor and
(iii) Area of cross section of the conductor. A rheostat/ variable resistor is used to change the resistance in a circuit.
(b) Given L = 50 m, A = 0,01 mm2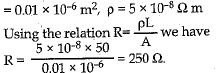
Q.30. (a) Draw magnetic field lines produced around a current carrying straight conductor passing through a cardboard. Name, state and apply the rule to mark the direction of these field lines.
(b) How will the strength of the magnetic field change when the point where magnetic field is to be determined is moved away from the straight wire carrying current ? Justify your answer.
OR
(a) Draw a schematic labelled diagram of domestic electric circuit.
(b) Why is it necessary to provide
(i) A fuse in an electric circuit.
(ii) An earth wire to electric appliance of metallic body? Explain. (5 Mark)
Ans: (a) Pattern of magnetic field lines produced around a current carrying straight conductor.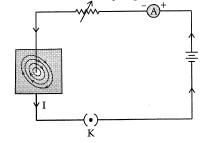
Right-hand thumb rule : If we are holding a current carrying straight conductor in right hand such that the thumb points towards the direction of current, then, the fingers will wrap around the conductor in the direction of the field lines of the magnetic field.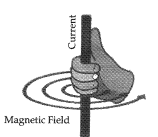
(b) As the compass is placed farther, deflection in the needle decreases. Thus, the magnetic field produced by given current decreases as the distance from it increases. The concentric circles around the wire become larger as we move away from it.
OR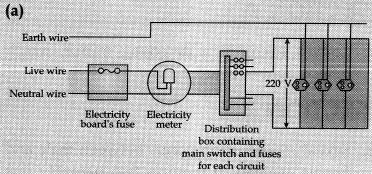
Fig. A schematic diagram of one of the common domestic circuits
(b) (i) It prevents damage to appliance due to overloading or short-circuiting.
(ii) Earth wire is connected to a metallic body buried deep inside Earth. It is used as safety measure.
It provides a low resistance conducting path for the current Any leakage of current to a metallic body does not give shock to user.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper (2019-20) - 4 - Science Class 10
| 1. What is the purpose of CBSE sample question papers for Class 10 science? |  |
| 2. How can CBSE sample question papers help in exam preparation for Class 10 science? |  |
| 3. Are CBSE sample question papers for Class 10 science similar to the actual board exam question paper? |  |
| 4. Can solving CBSE sample question papers guarantee good marks in the Class 10 science exam? |  |
| 5. Where can I find CBSE sample question papers for Class 10 science? |  |
















