Class 10 Science: CBSE Sample Question Paper (2019-20) - 2 | Science Class 10 PDF Download
Class-X
Science-086
SAMPLE QUESTION PAPER 2019-20
TIME: 3 Hrs.
M.M: 80
General Instructions:
1. The question paper comprises three sections - A, B and C. Attempt all the sections.
2. All questions are compulsory.
3. Internal choice is given in each section.
4. All questions in Section A are one - mark questions comprising MCQ, VSA type and
assertion-reason type questions. They are to be answered in one word or in one sentence.
5. All questions in Section B are three - mark, short - answer type questions. These are to be answered in about 50 - 60 words each.
6. All questions in Section C are five - mark, long - answer type questions. These are to be answered in about 80 - 90 words each.
7. This question paper consists of a total of 30 questions.
Section A
Q 1. How is the brain protected from shocks and injuries? (1 Mark)
Ans: The brain fits inside a bony box called skull, which protects brain. The brain is also protected by a fluid that provides further shock absorption.
Q.2. A uniform magnetic field exists in the plane of paper Electron Proton pointing from left to right as shown in the figure. In the field an electron and a proton move as shown. The electron and proton experience (1 Mark)
(a) forces both pointing into the plane of paper.
(b) forces both pointing out of the plane of paper.
(c) forces pointing into the plane of paper and out of the plane of paper respectively.
(d) forces pointing opposite and along the direction of the uniform magnetic field respectively.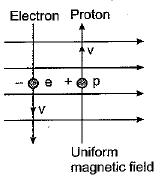
Ans: (a) On applying Fleming’s left-hand rule we find that both electron as well as proton experience forces pointing into the plane of paper.
Q.3. Answer question numbers 3(a) - 3(d) on the basis of your understanding of the following paragraph and the related studied concepts.
All of our energy comes from the sun, which is our nearest star. The sun sends out huge amounts of energy through its rays every day. We call this energy solar energy or radiant energy. Without the sun, life on earth would not exist, since our planet would be totally frozen.
We use this solar energy in many different ways. The sunlight lets us see and warms us.
Plants, use the light from the sun to grow. They store it as chemical energy. This process is called photosynthesis. The energy is stored in their roots, fruits, and leaves. This energy feeds every living thing on the earth. When humans and animals eat plants, and the food made from plants, we store the energy in our bodies, in our muscles and in our brain cells. We use this energy for everything we do. We use energy when we sing a song, think a thought, tell a joke, climb a ladder, make a pizza, or run a race. Everything needs energy!
Just as humans store energy in their bodies, the earth stores the sun's energy too.
The sun's energy is stored in coal, natural gas, water and wind. Coal, oil, and natural gas are known as fossil fuels.
Fossil fuels were formed over millions of years ago when the remains and fossils of prehistoric plants and animals sank to the bottom of swamps and oceAns: These animal and plant remains were slowly covered and crushed by layers of rock, mud, sand, and water. The pressure of all those layers caused the plants and animals to break, down and change into coal, oil and natural gas.
We use the energy in these fossil fuels to make electricity. We use electricity in many different ways. We light and heat our homes, schools and businesses using electricity, and to run computers, refrigerators, washing machines, and air conditioners. Our cars and planes nm on gasoline, which comes from oil. As of the year 2013, most of the energy we use comes from fossil fuels. However, fossil fuels are known as non-renewable sources of energy. They cannot be used over and oyer again. This means that one day they will run out! Luckily, there are some renewable energy sources we can use, that we can keep using. Unlike nonrenewable fossil, fuels, they will not run out. Three forms of renewable fuels are; solar (coming from the sun) energy, water energy and wind energy. Solar energy can be caught through solar cells and solar panels. People put solar panels on the top of houses to help capture the sun's energy and transform it into heat and electricity. Water is also used to produce electricity. Dams capture the energy of falling water and turn it into electricity. Wind is a third form of renewable energy. Wind turbines can capture the energy of the moving air and turn it into electricity. All these renewable energy sources are essential for us because they will not run out, so we need to get better and better at using them.
(a) Where does all of our energy come from? (1 Mark)
(b) How does the author describe renewable energy sources? (1 Mark)
(c) Most of the energy we use comes from fossil fuels. However, fossil fuels are known - as non-renewable sources of energy, so one day they will run out. (1 Mark)
(d) Based on the evidence in the passage, how can the sun best be described? (1 Mark)
Ans: (a) The sun
(b) Energy sources that will not run out.
(c) Renewable energy sources.
(d) crucial for life on earth
Q 4. Study the given table and answer the following questions. The table shows the pH value of the plaque surrounding the teeth of a child over 3 hrs.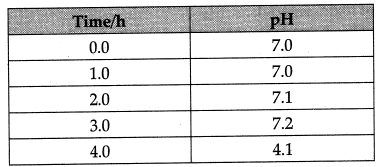
(a)The constituents of plaque are (1 Mark)
(i) Acid (ii) Saliva (iii) Bacteria (iv) AH of these
(b) What causes tooth decay? (1 Mark)
(c) State the time during the day when condition is most favourable for process of tooth decay, (1 Mark)
(i) 1.0
(ii) 2.0
(iii) 3.0
(iv) 4.0
(d) The nature of toothpastes commonly used to protect tooth decay is _____________ (1 Mark)
Ans:
(a) (iv) The constituents of plaque are acid, saliva, bacteria and food.
(b) A lower pH below 5.5, leads to tooth decay. At this pH, the calcium phosphate of enamel of tooth gets corroded.
(c) (iv) Time 4.0. Lowest pH indicate the highest amount of acid produced by the bacteria
(d) The tooth paste commonly used is basic so that the extra acid formed during tooth decay is neutralised and prevent tooth decay.
Q 5. Which one of the following juices secreted in the human body does not contain any enzyme? (1 Mark)
(a) Salivary juice
(b) Bile juice
(c) Gastric juice
(d) Pancreatic juice
Or
Which part of alimentary canal receives bile from the liver?
(a) Oesophagus
(b) Small intestine
(c) Stomach
(d) Large intestine Arts,
Ans: (b) Bile juice secreted by the liver in the human body does not contain any enzyme. Rather, the bile salts present in the bile juice breakdown fats into small globules increasing the efficiency of the action of the pancreatic enzymes.
Or
Small intestine receives bile from liver for breakdown of fats present in food.
Q.6. The properties related to metals are : (1 Mark)
(a) good conductors of heat
(b) good conductors of electricity
(c) malleable
(d) all the above
Ans: (d) all the above
Q 7. In an electrical circuit two resistors of 2Ω and 4Ω respectively are connected in series to a 6V battery. The heat dissipated by the 4Ω resistor in 5 s will be (1 Mark)
(a) 5 J
(b) 10 J
(c) 20 J
(d) 30 J
Ans: C
Q 8. In which of the following, the image of an object placed at infinity will be highly diminished and point sized? (1 Mark)
(a) Concave mirror only
(b) Convex mirror only
(c) Convex lens only
(d) Concave mirror, convex mirror, concave lens and convex lens
Ans: (d)
Explanation : The incident rays which comes from an object placed at infinity will be parallel and the rays parallel to the principal axis, after reflection/refraction by concave mirror, convex mirror, concave lens and convex lens, will pass or appear to pass through the principal focus . Hence, image will be highly diminished and point sized.
Q 9. A 10 mL solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl required to neutralise it will be (1 Mark)
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Ans:
(d) 16 mL (If we take double the amount of same NaOH solution, the amount of the same HCl solution required to neutralise it will, be double.)
Q.10. If yellow seeded wrinkled pea plant (YYrr) is crossed with green seeded round pea plant (yyRR), the seeds produced in F1 generation are (1 Mark)
(a) yellow and round
(b) green and round
(c) wrinkled and green
(d) wrinkled and yellow
Ans: (a)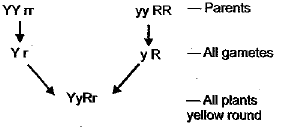
Q 11. With the increase in the concentration o f hydrogen ions, the pH value will : (1 Mark)
(a) Increase
(b) Decrease
(c) Remain constant
(d) Remain fluctuating
Ans: (a)
Q 12. Disposable plastic plates should not be used because (1 Mark)
(a) They are made of materials with light weight.
(b) They are made of toxic materials.
(c) They are made of biodegradable materials.
(d) They are made of non - biodegradable materials.
Ans: (d)
Explanation: Disposable plastic plates should not be used because they are made of non- biodegradable materials. Under certain conditions, the non-biodegradable substances can persist for a longer time and can also harm the various components of ecosystem.
Q 13. In each of the following questions, a statement of Assertion is given by the corresponding statement of Reason. Of the statements, mark the correct answer as (1 Mark)
Assertion When focal length of a lens increases, then its power decreases.
Reason Power of a lens is inversely proportional to focal length of lens.
(a) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are true, but Reason is not the correct explanation of Assertion.
(c) If Assertion is true, but Reason is false.
(d) If Assertion is false, but Reason is true.
Ans: (a) Power P of a lens is equal to reciprocal of the focal length f, i.e 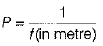
Thus, when focal length of a lens increases, then its power decreases. Hence, the option (a) is correct
TRUE OR FALSE: State whether the following statements are True or False :
Q.14. In graphite, each carbon atom is bonded to four other carbon atoms. (1 Mark)
Ans: False
Section B
Q.15. Write the chemical formula of plaster of Paris. How is it prepared? How is it different from gypsum? (3 Mark)
Ans: Chemical formula of plaster of Paris is 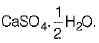
It is prepared by heating gypsum at 373 K. The reaction is as follows: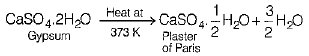
It contains only half mole of water of crystallisation whereas, gypsum contains two moles of water of crystallisation. In other words, piaster of Paris differs from gypsum in the number of moles of water of crystallisation.
Q.16. What are solar cells? Explain the structure of solar panel. List two principal advantages associated with solar cells. (3 Mark)
Ans: A solar cell is a device which converts solar energy directly into electricity.
A typical solar cell develops a voltage ranging from 0.5 V to I V and can produce about 0.7 watt of electricity. To produce high voltage and for delivering high power output, a large number of solar cells are connected in series. Such an arrangement is known as a 'solar panel’ and it can deliver enough electricity for practical use.
Two principal advantages associated with solar cells are as follows :
1. They have no moving parts and require little maintenance.
2. They can be set up even in remote areas.
Q.17. Which group of elements could be placed in Mendeleev's Periodic Table without disturbing the original order ? Give reason. (3 Mark)
Ans: Noble gases.
According to Mendeleev's classification, the properties of elements are the periodic function of their atomic masses and there is a periodic recurrence of elements with similar physical and chemical properties. Noble gas being inert, could be placed in a separate group without disturbing the original order.
Q 18. Trace the sequence of events which occur when a bright light is focused on your eyes. (3 Mark)
OR
List in tabular form three distinguishing features between autotrophic nutrition and heterotrophic nutrition.
Ans: When bright light is focused on our eyes, the Photoreceptors generate electric impulses and pass it to the sensory neurons. They carry the stimuli to the spinal cord which transports the message to brain. The brain sends the response to the muscles of the eyelids to close by contracting the pupil.
Receptor → Sensory neuron → Spinal cord → Brain → Motor neuron → Eye → Contraction of eye muscles
OR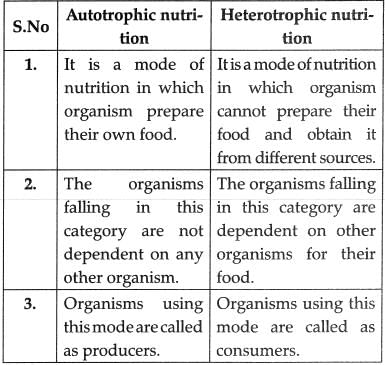
Q 19. Obtain an expression for the heat produced in a conductor of resistance R , when current I is flowing through it. (3 Mark)
Or
(i) Give the function of the earth wire in electrical instruments. Why is it necessary to earth the metallic electric appliances?
(ii) What are the harmful effects of overloading in an electric supply? When does it occur? (iii) What is the capacity of fused wire in the line to feed
(a) lights and fans, (b) appliances of 2 kW or more power?
Ans:
W= VQ ..(i)
But, current flowing through the conductor is given by I = Q/t
⇒ Q = It ...(ii)
From Eqs. (i) and (ii), we get W = V/t ...(iii)
By Ohm ’s law, V = IR ..(iv)
Now, from Eqs. (iii) and (iv), we have
W = IR.I.t
W = I2Rt
Assuming that all the electrical work done or all the electrical energy consumed is converted into heat energy, H = W = l2Rt
or
(i) The function of earth wire in electrical instruments is to prevent them from damage and to save the users from fatal electric shock. This is because, earthing provides a low resistance conducting path for current. Hence, in case of leakage of current in'metallic body of appliance, it will be discharged to earth. Thus, it is necessary to earth the metallic appliances to protect them against sever damage and the user from electric shock.
(ii) The harmful effect of overloading are as follows:
(a) It may damage electrical appliances.
(b) It causes the overloading in the circuit, it may lead to fire because of excessive heating due to passing of large amount of current.
(iii) (a) 5A (b) 15A
Q.20. What is a homologous' series of carbon compounds ? Give an example and list its three characteristics. (3 Mark)
Ans: A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series. The molecular formula of any two successive members differs by - CH2.
For example methane (CH4) and ethane (C2H6 ) are two members of homologous series of alkanes.
Characteristics:
(i) They have the same general formula.
(ii) Difference in molecular mass of two successive members is 14 u.
(iii) They have similar chemical properties.
Q.21. How does chemical coordination occur in plants ? Explain with the help of three examples. (3 Mark)
Ans: In plants, chemical coordination occurs through various phytohormones.
(i) Auxins secreted by growing tissues. They provide growth of plants.
(ii) Gibberelins cause stem elongation, seed germination and flowering.
(iii) Cytokinins present in areas of actively dividing cells like fruits, seeds. Promote cell division.
(iv) Abscisic acid inhibits growth, respond to environmental stress, (any three)
Q 22. It is necessary to connect an earth wire to electric appliances having metallic covers. Why? How will you identify earth wire in household circuit ? (3 Mark)
Ans: The Earth wire is connected to a metallic plate deep inside the Earth. In this way, the metallic body of appliance is connected to the Earth, which provides a low resistance conducting path for electric current.
Hence, any leakage of current to the metallic body of appliance keeps the potential to that of Earth. The user might not get a severe electric shock on touching such an appliance.
Earth wire has green insulation, so that it can be identified.
Q 23. (i) Why do stems grow towards the source of light? (3 Mark)
(ii) Name the plant hormone which
(a) inhibits growth
(b) promotes cell division
(c) helps the cells to grow longer
(d) helps in the growth of stem
or
Answer the following.
(i) Which endocrine gland is present in males but not in females? (ii) Name the endocrine gland associated with kidneys.
(iii) Which gland secretes both digestive enzymes as well as hormone.
Ans:
(i) The stems grow towards the source of light due to the accumulation and diffusion of auxin on the shaded or lower side of the shoot apex. Hence, more growth is observed on the shaded side and the stem grows upward.
(ii)
(a) Abscisic acid inhibits growth.
(b) Cytokinin promotes cell division.
(c) Auxin helps the cells to grow longer.
(d) Gibberellin helps in the growth of stem and flower.
or
(i) Testes are the endocrine glands present only in males and secrete male sex hormones, bringing about changes associated with puberty in males.
(ii) Adrenal glands are located on the top of the two kidneys and secrete adrenaline hormone when the body is under duress.
(iii) Pancreas is the digestive gland which secretes pancreatic juice for digestion of proteins, fats and insulin hormone for regulating the glucose level in blood.
Q.24. Write the essential function performed by ozone at the higher levels of the Earth's atmosphere. How is it produced ? Name the synthetic chemicals mainly responsible for the drop of amount of ozone in the atmosphere. How can the use of these chemicals be reduced ? (3 Mark)
Ans: Ozone layer prevents harmful ultraviolet radiations to reach earth’s atmosphere.
Ozone is formed at the higher level of atmosphere by the action of UV radiations on 02 molecule. High energy UV radiations split apart some oxygen molecules into atomic oxygen which react with molecular oxygen to form ozone molecules as shown by the following equations :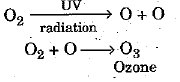
Chlorofluorocarbons are responsible for the depletion of ozone layer. These compounds which are used in refrigerators and fire extinguishers get leaked during manufacture or repair and go up high into the atmosphere.
To reduce the use of these chemicals, we should
(i) Reduce the use of ACs, coolants, aerosols etc.
(ii) Recycle these gadgets or discard them carefully.
(iii) It has been found that using AC at 25 °C reduces the electricity consumption and increases the life of an AC.
(iv) Be more environment friendly in our day to day living.
SECTION C
Q 25. Ankit's teacher taught him that consumption of small quantities of dilute ethanol causes drunkenness. Intake of even a small quantity of pure ethanol can be lethal. Also, long-term consumption of alcohols lead to many health problems. (5 Mark)
(i) What is ethanol?
(ii) State its two properties.
(iii) What happens, when it is h e ated w ith excess o f cone. H2SO4 at 443 K?
(iv) What role does cone. H2SO4 play in this reaction?
(v) Write the chemical equation of the reaction involved and the structural formula of the main product formed.
Or
A sweet smelling organic compound (X) having the molecular formula C5H10O2 , on treatment with sodium hydroxide (NaOH) gives a sodium salt of a carboxylic acid, which upon acidification gives 2-methyl propanoic acid and an alcohol (Y). Intake of alcohol (F) in very small quantities can cause blindness and finally death. Alcohol (Y) upon complete oxidation gives a carboxylic acid (Z), which is found in red ants.
(i) Write the structural formula and chemical name of the organic compound (X).
(ii) What is the name of the alcohol (Y)?
(iii) Name the carboxylic acid (Z), which is found in red ants.
(iv) Which functional group is present in the compound, which is formed upon oxidation of alcohol (Z) in the liver?
(v) Write a balanced chemical equation when the organic compound (X) is combined with sodium hydroxide (NaOH) solution.
Ans:
(i) Ethanol is an alcohol, its common name is ethyl alcohol and chemical formula is CH3CH2OH.
(ii) Two properties of ethanol are :
(a) It is a colourless, volatile liquid having pleasant smell and burning taste.
(b) It is soluble in water due to its ability to form H-bonds with water molecules.
(iii) When ethanol is heated at 443 K (170°C) with excess of cone, sulphuric acid (H2SO4), water molecule is removed and ethene is formed. Thus, dehydration takes place.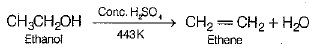
(iv) Role of cone. H2SO4 In this reaction, cone. HgS04 acts as a dehydrating agent ( a substance which is used to remove water molecule).
(v) Structural formula of the main product, i.e. ethene is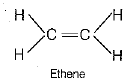
Or
For the given conditions, reaction takes place as follows: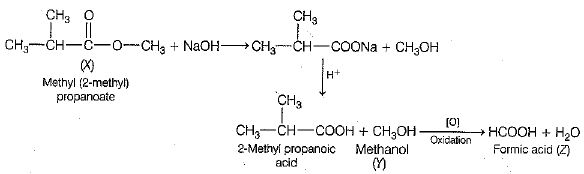
(i) Structural formula of organic compound (X) is 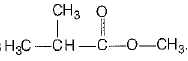
its chemical name is methyl (2- methyl) propanoate
(ii) Alcohol (V) is methanol (CH3OH).
(iii) Carboxylic acid (Z) is formic acid (HCOOH), which is found in red ant.
(iv) In the liver, methanol (CH3OH) is partially oxidised to methanal (HCHO). It contains aldehyde group — CHO as a functional group
(V) 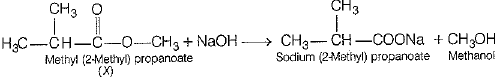
Q.26. (a) How will you in fer with the help of an experiment that the same current flows through every part of a circuit containing three resistors in series connected to a battery ? (b) Consider the given circuit and find the current flowing in the circuit and potential difference across the 15 Ω resistor when the circuit is closed. (5 Mark) 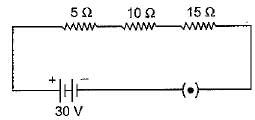
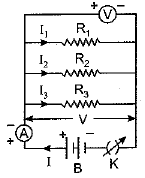
Ans: (a) We take three resistors R1, R2 and R3 and join them in series between the points X and Y in an electric circuit as shown here.
Plug the key and note the ammeter reading. Then change the position of ammeter to anywhere in between the resistors and again note the ammeter reading. We find that ammeter reading remains unchanged. It shows that in series arrangement same current flows through each resistor.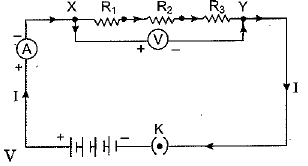
(b) Here R1 = 5Ω ,R2=10Ω,R3 = 15 Ω and V= 30 V
As the three resistances are connected in series, the net resistance
R =R1 + R2+ R3 = 5 + 10 + 15 — 30 Ω
∴ Current flowing in the circuit 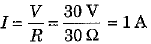
and Potential difference across R3 of 15 Ω, V3 = IR3 = 1 x 15 = 15 V
Or
(a) Three resistors R1, R2 and R3 are connected in parallel and the combination is connected to a battery, ammeter, voltmeter and key. Draw suitable circuit diagram and obtain an expression for the equivalent resistance of the combination of the resistors.
(b) Calculate the equivalent resistance of the following network :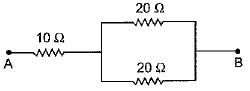
Ans: (a) Circuit diagram of three resistances in a parallel grouping is shown here. Here one ends of all the resistances are joined at one point and the other ends of all the resistances are joined at another point. The cell providing current is joined across these two points.
It is obvious that, in parallel combination of three resistances R±, R2 and R3, the current in each of the three resistances is different. If I is the current drawn from the cell then it is divided into branches I1, I2 and I3. Thus, I = I1 + I2 + I3.
However, the potential difference across each of these resistances is the same as the voltage V of the cell.
Thus, from Ohm’s law 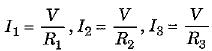
If R is the equivalent resistance then,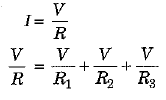
and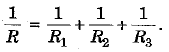
Thus, in parallel grouping reciprocal of the equivalent resistance is equal to the sum of the reciprocals of the individual resistances.
(b) In the given network, two resistances R1 = 20 Ω and R2 = 20 Ω are joined in parallel. So th e ir combined resistance R12 is given as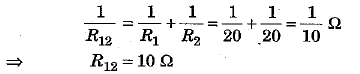
Now R12 = 10 Ω is connected in series with a resistance R3 = 10 Ω.
Hence, equivalent resistance of the network R = R12 + R3 = 10 + 10 = 20 Ω.
Q 27. (a) Draw a sectional view of the human heart and label on it Aorta, Pulmonary arteries, Vena cava, Left ventricle.
(b) Why is double circulation of blood necessary in human beings ? (5 Mark)
Ans: (a)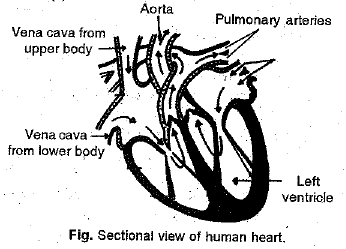
b) Double circulation of blood is necessary to :
(i) separate deoxygenated blood from oxygenated blood.
(ii) meet high energy and oxygen demands.
(iii) maintain constant body temperature
Q 28. (a) Draw a diagram of hum an excretory system and label the following parts on it : (5 Mark)
(i) Right Renal Artery
(ii) Vena cava.
(iii) Urinary bladder
(iv) Left kidney
(b) List two vital functions of kidney.
Ans: (a) Human Excretory system: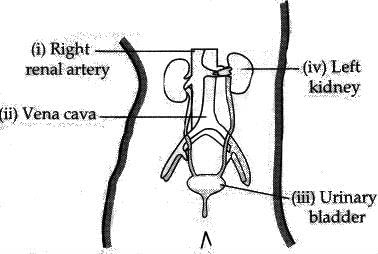
(b) Vital functions of kidney:
(i) To regulate right amount of water in body, like urea from blood,
Q. 29. Answer the following questions based on the diagram given below (5 Mark) 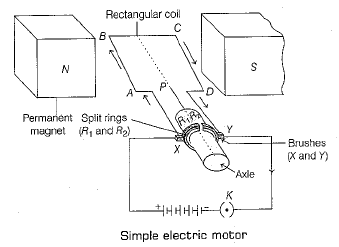
(i) Explain working of electric motor.
(ii) In what way this simple electric motor is different from commercial motor?
(iii) How the speed of rotation of motor be increased
or
(i) Justify the following statements.
(a) Two magnetic field lines never intersect each other.
(b) Magnetic field lines are closed curves.
(ii) A permanant electromagnet using a solenoid is as shown below 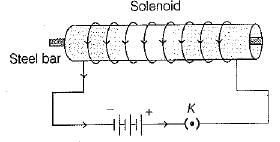
Under what conditions permanent electromagnet is obtained, if a current carrying solenoid is used?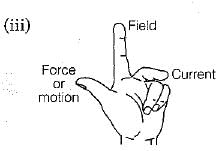
From the above figure identify the rule and state one application of this rule.
Ans: Working
(i) Let coil ABCD be in horizontal position. When the key is switched on, then the current flows in the coil ABCD through brush Y via split ring R1, and flows back to battery through the brush Y via split ring R2.
(ii) Applying, Fleming’s left hand rule, no force acts on arm BC and AD as they are parallel to the magnetic field, arm AB experiences a force in downward direction and arm CD experiences an equal force in upward direction. Thus, a torque acts on the coil and it rotates in anti-clockwise direction.
(iii) While rotating, the coil reaches the vertical position, brushes loose contact with the rings and current stops flowing. But the coil does not stop due to inertia of the motion.
(iv) When the coil passes the vertical position, then the rings automatically change their positions and come in contact with opposite brushes.
(v) This reverses the direction of a current through the coil ABCD but the direction of current in external circuit remains same.
(vi) So, the force on right hand side acts always in upward and a force on left hand side is always in downward direction. Thus, the coil continues to rotate in anti-clockwise direction.
A commercial electric motor is one which uses the following :
(i) An electromagnet in place of permanent magnet.
(ii) Large number of turns of the conducting wire of current carrying coil.
(iii) A soft iron core on which the coil is wound. The combination of soft iron core and coil is known as an armature. It enhances the power of motor.
Thus, the commercial electric motors do not use permanent magnet to rotate the armature because permanent magnets are weak and do not produce strong magnetic field in the region.
The speed of rotation of the motor can be increased by
(i) increasing the strength of the current in the coil or increasing the number of turns in the coil.
(ii) increaseing the area of the coil, increasing the strength of magnetic field.
Or
(i) (a) A tangent drawn on any point on magnetic field line indicates the direction of force. Then, if two lines intersect each other at a point, it means that force is acting in two directions at that point, i.e. there are two directions of the magnetic field at the point of intersection which is not possible at the same time. Thus, the two magnetic field lines can never intersect each other,
(b) The direction of field lines outside a magnet is from North-pole to South-pole while it Is from South to North inside the magnet and thus forms closed curves.
(ii) The conditions to obtain permanent electromagnet, if a current carrying solenoid is used are as follows:
(a) The current flowing through the solenoid should be direct current.
(b) The rod inside solenoid should be made up of a magnetic material such as steel.
(c) The magnetic material should be of high retentivity and should not lose its magnetism easily.
(iii) From the given diagram, the rule associated is Fleming's left hand rule.
One application of Fleming's left hand rule is electric motor which is used in electric devices like fan, grinder, etc.
Q.30. (a) What are homologous structures ? Give an example.
(b) “The sex of a newborn child is a matter of chance and none of the parents may be considered responsible for it.” Justify this statement with the help of a flow chart showing sex-determination in human beings. (5 Mark)
Ans: (a) Homologous organs: Such organs which may have different functions but similar structure and origin. Example, fore arm of frog, lizard, bird and human.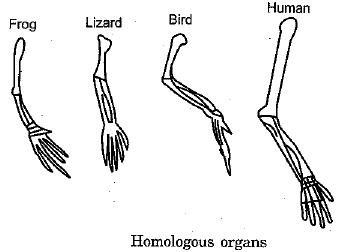
(b) Sex of the child is a matter of chance. This is because females produce only one type of gamete - carry X chromosome. While males produce two types of gametes — 50% that carry X chromosome while 50% that carry Y chromosome.
It's a matter of chance as to which male gamete will fertilise the egg and decide the sex of the child.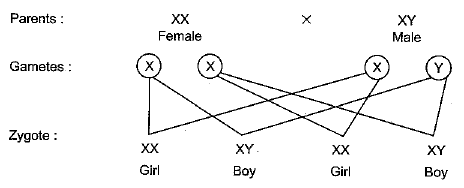
Hence, it is seen that there are 50 : 50 chances of a couple having a son or a daughter.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper (2019-20) - 2 - Science Class 10
| 1. What is the CBSE Sample Question Paper for Class 10 Science? |  |
| 2. How can the CBSE Sample Question Paper for Class 10 Science help students? |  |
| 3. Are the questions in the CBSE Sample Question Paper for Class 10 Science similar to the actual board exam? |  |
| 4. Can solving the CBSE Sample Question Paper for Class 10 Science guarantee good marks in the board exam? |  |
| 5. Where can I find the CBSE Sample Question Paper for Class 10 Science? |  |
















