Class 10 Science: CBSE Sample Question Paper (2019-20) - 8 | Science Class 10 PDF Download
Class-X
Science-086
SAMPLE QUESTION PAPER 2019-20
TIME: 3 Hrs.
M.M: 80
General Instructions:
1. The question paper comprises three sections - A, B and C. Attempt all the sections.
2. All questions are compulsory.
3. Internal choice is given in each section.
4. All questions in Section A are one - mark questions comprising MCQ, VSA type and
assertion-reason type questions. They are to be answered in one word or in one sentence.
5. All questions in Section B are three - mark, short - answer type questions. These are to be answered in about 50 - 60 words each.
6. All questions in Section C are five - mark, long - answer type questions. These are to be answered in about 80 - 90 words each.
7. This question paper consists of a total of 30 questions.
Section A
Q 1. Name the method by which Hydra reproduces. Is this method sexual or asexual ?
OR
Name the respiratory pigment in human beings. Where is this pigment found ? (1 Mark)
Ans:
Hydra normally reproduces by budding.
It is an asexual reproduction.
or
The respiratory pigment in human being is haemoglobin. Haemoglobin is present in RBC's of blood in humAns:
Q 2. What change will you observe if you test soap with litmus paper (red and blue) ? (1 Mark)
Ans: Soaps are alkaline, so it will turn red litmus blue and, no effect on blue litmus paper.
Q.3. Resistivity of a given conductor depends on (1 Mark)
(а) length of conductor.
(b) length as'well as cross-section area of conductor.
(c) material and dimensions of conductor.
(d) material of conductor and temperature.
Ans: (d) Resistivity of a given conductor depends only on its material and the temperature. Resistivity does not depend on the dimensions of the conductor.
For Q.Nos. 4(i)-4(iv) are based on the table given below. Study these table related to answer the questions that follow :
Q 4. A few biodegradable and non-biodegrable substances are given below
(i) Arrange them in group A and group B as degradable and non-biodegradable substances, respectively. (1 Mark)
(ii) What are biodegradable substances? (1 Mark)
(iii) We classify different substance as non-biodegradable when
(a) they have a tendency to persist for long time
(b) they are toxic and not metabolised
(c) not decomposed by microbes
(d) All of the above (1 Mark)
(iv) Biodegradable wastes usually do not pollute the environment. They are considered a threat only when (1 Mark)
(a) they are present in small amounts
(b) their amount is large
(c) they cannot be degraded in nature at right time
(d) Both (b) and (c)
Ans:
(i)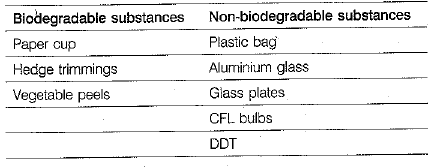
(ii) The substances which can be broken down to simpler forms are known as biodegradable wastes.
(iii) (c) When a substance cannot be broken down by microbial action to simpler forms, it is classified as non-biodegradable. They persist in nature for very long time and pollutes it.
(iv) (d) Biodegradable wastes pollute the environment only their amount is large which cannot be degraded into harmless forms in nature at the right time.
Q 5. Common salt besides being used in kitchen can also be used as the raw material for making: (1 Mark)
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
(a) (i) and (ii)
(b) (i), (ii) and (iv)
(c) (i) and (iii)
(d) (i), (iii) and (iv)
OR
While studying the saponification reaction, what do you observe when you mix an equal amount of colourless vegetable oil and 20% aqueous solution of NaOH in a beaker?
(a) The colour of the mixture has become dark brown.
(b) A brisk effervescence is taking place in the beaker.
(c) The outer surface of the beaker has become hot.
(d) The outer surface of the beaker has become cold.
Ans: c
Explanation: The common salt obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda and many more.
OR
Correct Option (c)
Explanation: The beaker becomes hot because it is an exothermic reaction.
Q 6. Electrical resistivity of a given metallic wire depends upon (1 Mark)
(a) its length
(b) its thickness
(c) its shape
(d) nature of the materia
Ans: (d)
Q 7. Ketones possess the functional group - (1 Mark)
(a) 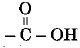
(b) 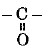
(c) 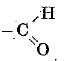
(d) - OH
Ans: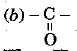
Q 8. Far point for a normal eye is equal to (1 Mark)
(a) 25 cm
(b) infinity
(c) greater than 25 cm and less than 100 cm
(d) less than 25 cm
Or
The human eye can focus oject at different distances by adjusting the focal length of eye lens. This is due to
(a) presbyopia
(b) accommodation
(c) near sightedness
(d) far sightedness Arts,
Ans: (b) Far point for a normal eye is infinity while near point for normal eye is 25 cm.
Or -(b) Accommodation is the ability of eye lens to focus both near and distant objects by adjusting its focal length.
Q 9. An optical device has been given to a student and he determines its focal length by focusing the image of the Sun on a screen placed 24 cm from the device on the same side as the Sun. Select the correct statement about the device. (1 Mark)
(a) Convex mirror of focal length 12 cm
(b) Convex lens of focal length 24 cm
(c) Concave mirror of focal length 24 cm
(d) Convex lens of focal length 12 cm
Ans: (c)
Explanation: The optical device is a concave mirror. It is because when light rays from a distant object (here it is Sun) fall on this mirror, its image Is formed at the focus of the mirror on the same side as the object. Thus, in the given case, distance between the mirror and the screen is the focal length of the concave mirror. Hence, the focal length of the given device (concave mirror) is 24 cm.
Q 10. Calcium oxide reacts vigorously with water to produce: (1 Mark)
(a) Calcium hydroxide releasing a large amount of heat
(b) Calcium hydroxide absorbing a large amount of heat
(c) Calcium oxide and hydrogen with a release of large amount of heat.
(d) Calcium oxide and hydrogen with the absorption of large amount of heat
Ans : (a)
Complete the following statements with appropriate answer(s) in the blank space is):
Q 11. Speed of light in diamond is _________ because its refractive index is _________ in nature. (1 Mark)
Ans: minimum, maximum
Q 12. At the time of short circuit, the current in the circuit (1 Mark)
(a) vary continuously
(b) increases heavily
(c) decreases heavily
(d) remains same
Ans:
(b) When live wire and neutral wire come in contact with each other, then it is known as shon circuiting. In this case, the resistance of the circuit is almost zero, which results in the flow of heavy current.
Q 13. Assertion (A) : Unisexual flowers have separate male and female flowers.
Reason (R) : Cucumber, pumpkin and watermelon are example of unisexual flowers.
OR
Assertion (A) : Units which make up the nervous system are called neurons.
Reason (R) : Nerve impulses are carried by dendrites towards the cell body (1 Mark)
Ans:
Correct option: (b)
Explanation: Unisexual flowers have separate male and female flowers. The example includes cucumber, pumpkin and watermelon.
OR
Correct option: (b) Explanation : Both the statements are true. Nervous system is the system of conducting tissues that receives the stimulus and transmits it to other parts of the body forming a network of nerves. It is involved in receiving information (sensation) and generating responses to that information (motor response). The units which make up the nervous system are called nerve cells or neurons. Nerve impulses are always transmitted across a synapse from the axon terminals of one neuron to the dendrite /cell body of the next neuron.
Q 14: Assertion: A convex lens conform both real and virtual images.
Reason : When an object is placed between the optical centre and focus of a convex lens a virtual image is formed. (1 Mark)
(i) A
(ii) B
(iii) C
(iv) D
Ans: (i) Both A and R are true and R is correct explanation of the assertion
Section B
Q 15. Based on the group valency of elements, write the molecular formula of the following compounds giving justification for each: (3 Mark)
(i) Oxides of first group elements.
(ii) Halides of the elements of group 13, and
(iii) Compounds formed when an element A of group 2 combines with an element B of group 17
Ans: (i) Valency of group 1 element is 1. Valency of oxygen is 2.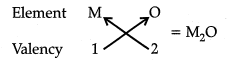
So, the molecular formula of oxide of first group elements is M20 where M is the group element and O is oxygen.
(ii) Valency of group 13 elements is 3.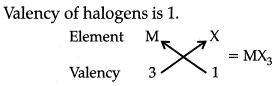
So, the molecular formula of halide of group thirteen elements is MX3 where M is the group 13 element and X is halogen.
(iii) Valency of group 2 elements is 2.
Valency of group 17 elements is 1.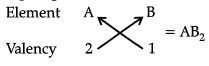
So, the molecular formula is AB2.
Where A is the group 2 element and B is the group 17 element.
Q.16. What will be the action, of the following substances on litmus paper ?
DryHClgas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution. (3 Mark)
Ans: 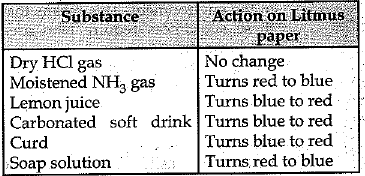
Or
Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.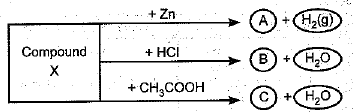
Ans:
X - NaOH (Sodium hydroxide)
A - Na2Zn02 (Sodium zincate)
B - NaCl (Sodium chloride)
C - CH3COONa (Sodium acetate)
Q.17. On heating blue coloured powder of copper (II) nitrate in a boiling tube, black copper oxide, O2 and a brown gas X is formed. (3 Mark)
(a) Identify the type of reaction and the gas X.
(b) Write balanced chemical equation of the reaction.
(c) Write the pH range of aqueous solution of the gas X.
Ans: (a) It is a decomposition reaction. The gas X evolved is N02 (Nitrogen dioxide).
(b) The balanced chemical equation is as under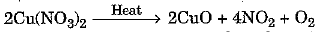
As the solution is acidic, the range is 0 - 6.9 (less than 7).
Q 18. In evolutionary terms, can we say that one organism has a better body design than the other? Why or why not? (3 Mark)
Ans: Evolution is the generation o f diversity due to environmental selection. More and more body designs have emerged overtime. There is actually no real ‘progress' in the idea of evolution. Generation of more complex body designs does not mean that the older designs are inefficient. The older and simpler designs still survive on the Earth. One of the simplest life forms, i.e. bacteria are found in the most inhospitable habitats like deep thermal vents, hot springs, etc. Hence, it is not fair to say that one organism has a ‘better’ body design than others.
Q 19. What are plant hormones ? Name the plant hormones responsible for the following: (3 Mark)
(i) Growth of stem
(ii) Promotion of cell division
(iii) Inhibition of growth
(iv) Elongation of cell
Ans: Plant hormones or phytohormones are plant growth substances which are secreted in small quantities and are involved in growth promoting activities such as cell division, cell enlargement, pattern formation, flowering, fruiting, tropic growth and seed formation,
(i) Gibberellins
(ii) Cytokinins
(iii) Abscisic add
(iv) Auxins
Q 20. A cross was carried out between a pure bred tall pea plant and a pure bred dwarf pea plant and F1 progeny was obtained, hater, the F2 progeny was selfed to obtain F2 progeny. Answer the following questions: (3 Mark)
(a) What is the phenotype of the Ft progeny and why ?
(b) Give the phenotypic ratio o f the F2 progeny.
(c) Why is the F2 progeny different from the F1 progeny ?
Ans: (a) Tall, because genes responsible for tallness are dominant over dwarf trait.
(b) 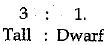
(c) Because in F1 generation, recessive genes got expressed in homozygous condition.
Q.21. What is transpiration ? last its two functions. (3 Mark)
Ans: Transpiration is loss of water in the form of water vapours from the upper parts of the plants, mainly leaves.
Its function are :
(i) Transpiration creates transpiration pull for absorption and transport of water up through the xylem in plants.
(ii) It supplies water for photosynthesis.
(iii) It transports minerals from the soil to all parts of the plant.
(iv) As evaporation causes cooling, transpiration assists in cooling of leaves. Not all the solar radiations absorbed by leaves are used in photosynthesis, but some will cause heating of leaves. Transpiration however reduces the heating of leaves.
(v) Transpiration maintains the shape and structure of the plants by keeping the cells turgid.
(vi) Transpiration removes excess amount of water.
Or
(a) What is translocation ? Why is it essential for plants ?
(b) Where do the substances in plants reach as a result of translocation ?
Ans: (a) Translocation is defined as the transport of soluble organic molecules, formed as a product of photosynthesis in the form of glucose, from one part of the plant to another.
By this process, all plant parts get nutrition, hence it is essential.
(b) These organic molecules reach all. the parts of the plant like roots, fruits, seeds — for storage, stem, flowers - for growth.
Q 22. If two resistors of resistance 5Ω and 10Ω respectively, are connected to a battery of emf 6 V, then determine the value of the total resistance and current in the circuit when, they are connected in (3 Mark)
(i) parallel combination,
(ii) series combination.
Ans:
Given, R1 = 5Ω, R2 = 10Ω, V = 6 V
(i) For parallel combination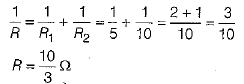
∴ Total current in the circuit 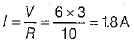
(ii) For series combination R = R1 + R2 = 5 + 10 = 15Ω
∴ Total current in the circuit 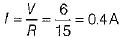
Q 23: List three advantages each of : (3 Mark)
(i) exploiting resources with short term aims, and
(ii) using a long term perspective in managing our natural resources
Ans: (i) Advantages of exploiting resources with
short-term alms:
1. Beneficial for the present generation.
2. Growth of economy at a faster rate.
3. Comfort will increase.
(ii) Advantages of long term perspective in managing our natural resources :
1. Resources can be used efficiently by present as well as future generations.
2. Sustainable management of resources.
3. Pollution and degradation of environment will be less.
Q 24. A person needs a lens of power -4.5 D for correction of her vision.
(a) What kind of defect in vision is she suffering from?
(b) What is the focal length of the corrective lens?
(c) What is the nature of the corrective lens
or
How will you use two identical prisms so that a narrow beam of white light incident on one prism emerges out of the second prism as white light? Draw the diagram (3 Mark)
Ans:
(a) Myopia
(b) f = 1/P = 1/-4.5 = - 2/9 = - 0.22 m
(c) Concave len
or
The second prism should be placed in the inverted position with respect to the first. This is shown in the diagram below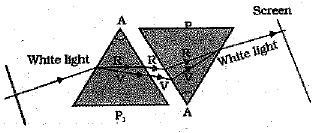
Section C
Q 25. (a) Explain why carbon forms covalent bond ? Give two reasons for carbon forming a large number of compounds.
(b) Explain the formation of ammonia molecule. (5 Mark)
Ans: (a) Carbon has electronic configuration 2, 4. It could gain four electrons forming C-4 anion or lose 4 electrons to form C+4 cation. Both are not possible due to energy considerations.
Carbon overcomes this problem by sharing electrons and forming covalent compounds.
Two masons for forming large number of compounds am:
1. Catenation
2. Tetra valency'
(b) Formation of NH3 molecule
N -2, 5
H - 1
Three hydrogen atoms each share their 1 electron with nitrogen to form three covalent bonds and make an ammonia molecule (NH3).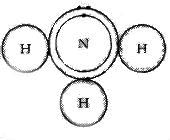
Q.26. (a) What are hydrocarbons ? Give examples,
(b) Give the structural differences between saturated and unsaturated hydrocarbons with two examples each.
(c) What is a junctional group ? Give examples of four different functional groups. (5 Mark)
Ans: (a) Compounds of carbon and hydrogen are called hydrocarbons. Examples, methane, ethane etc.
(b) Saturated hydrocarbons contain carbon-carbon single bonds.
Unsaturated hydrocarbons contain atleast one carbon-carbon double or triple bond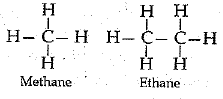
Saturated hydrocarbon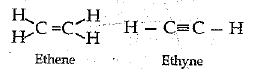
Unsaturated hydrocarbons
(c) Functional group : An atom/ group of atoms joined in a specific manner which is responsible for the characteristic chemical properties of the organic compounds. Examples are hydroxyl group (“OH), aldehyde group (—CHO), carboxylic group (— COOH) etc.
Q.27. What is transpiration ? last its two functions. (5 Mark)
Ans: Transpiration is loss of water in the form of water vapours from the upper parts of the plants, mainly leaves.
Its function are :
(i) Transpiration creates transpiration pull for absorption and transport of water up through the xylem in plants.
(ii) It supplies water for photosynthesis.
(iii) It transports minerals from the soil to all parts of the plant.
(iv) As evaporation causes cooling, transpiration assists in cooling of leaves. Not all the solar radiations absorbed by leaves are used in photosynthesis, but some will cause heating of leaves. Transpiration however reduces the heating of leaves.
(v) Transpiration maintains the shape and structure of the plants by keeping the cells turgid.
(vi) Transpiration removes excess amount of water.
Or
(a) What is translocation ? Why is it essential for plants ?
(b) Where do the substances in plants reach as a result of translocation ?
Ans: (a) Translocation is defined as the transport of soluble organic molecules, formed as a product of photosynthesis in the form of glucose, from one part of the plant to another.
By this process, all plant parts get nutrition, hence it is essential.
(b) These organic molecules reach all. the parts of the plant like roots, fruits, seeds — for storage, stem, flowers - for growth.
Q 28. ‘The sexual act always has the potential to result in pregnancy'. What approach would you use to prevent pregnancies? (5 Mark)
Or What would result if fertilisation takes place in humans? Also, incorporate the post-fertilisation changes.
Ans: The prevention of pregnancy is called contraception or birth control. The methods of contraception are summarised in the following table
Methods of Birth Control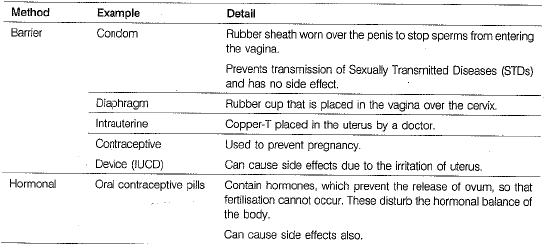

or
The fusion of the nucleus of the sperm (male gamete) and ovum (female gamete) is known as fertilisation This process occurs only when both the sperm and an egg is present in the oviduct at the same time. The sperm enters into the vagina by the process of copulation or mating. The sperms are highly active and motile. They swim into the uterus through cervix and then pass into the oviduct. When a sperm reaches the egg, it penetrates the ovum. Syngamy or fusion of male and female nuclei occurs to form a zygote. The zygote undergoes many cycles of cell division to form an embryo.
The embryo sinks down and reaches into the soft and thick lining of the uterus. The embedding of thr embryo in the thick lining of the uterus is known as implantation.
During pregnancy, the placenta grows into a disc between the uterine wall and the embryo. Placenta form: finger-like projections called villi towards embryo, which provide a large surface area for the exchange o m itrifintc; and waste nrndunts between the mother and the develoDina embrvo.
Q 29. An object is placed at a distance of 60 cm from a concave lens of focal length 30 cm.
(i) Use lens formula to find the distance of the image from the lens.
(ii) List four characteristics of the image (nature, position, size, erect/inverted) formed by the lens in this case.
(iii) Draw ray diagram to justify your answer of part (ii).
OR
(i) 4.5 cm needle is placed 12 cm away from a convex mirror of focal length 15 cm. Give the location of the image and the magnification.
Describe what happens as the needle is moved further from the mirror.
(ii) What kind of mirror is used in a solar furnace ? Give reason for using this mirror
(iii) One half of a convex lens is covered with a black paper. Will this lens produce a complete image of the object ? justify your answer. (5 Mark)
Ans:
(i) Given u = - 60 cm, f = -30 cm
Using lens formula,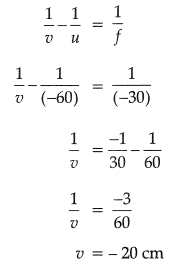
(ii) Distance of the image will be 20 cm in front of lens.
(ii) Nature: Virtual
Position: Between F1 and O or on same side of lens where object is placed.
Size : Diminished and 1/3 rd of object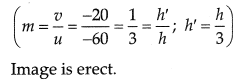
(iii)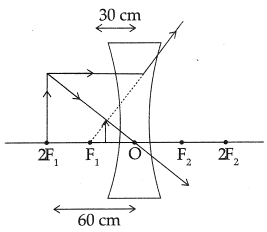
or
(i) u = -12 cm,
f = + 15 cm
Using mirror formula,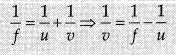
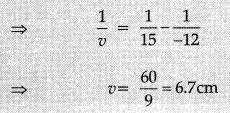
Magnification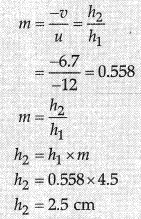
As the needle moved farther from the mirrot image to the focus and size of the image goes on decreasing
(ii) Concave mirrors are used in solar furances, as they concentrate solar in the focal plane and help in attaining high tempratures.
(iii) When one half of a convex lens is covered with a black paper, the lens will produce the complete image of the object of the object, but the intensity of the image is reduced because rays only from the top portion of the lens are regracted and form the image.
Q 30. Draw ray diagrams showing the image formation by a concave lens when an object is placed.
(a) at the focus of the lens
(b) between focus and twice the focal length of the lens
(c) beyond twice the focal length of the lens
Or
(a) State laws o f refraction.
(b) A ray of light enters from a medium A into a slab made-up of a transparent substance B (as shown in the figure).
Refractive indices of medium A and B are 2.42 and 1.65 respectively. Complete the path o f ray of light till it emerges out of the slab. (5 Mark)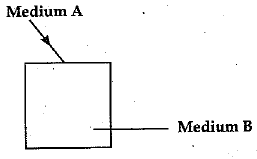
Ans:
The diagrams are as shown.
(a) Object placed at the focus.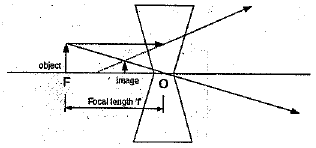
(b) between focus and twice the focal length of the lens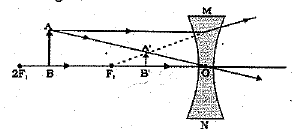
(c) beyond twice the focal length of the lens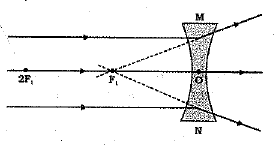
or
(a) The two laws are:
(i) The incident ray, the refracted ray and the normal to the interface of two transparent media at the point of incidence, all lie in the same plane.
(ii) The ratio of sine: of angle of incidence to the sine of angle of refraction is a constant, for the light of a given colour and for the given pair of media. This law is also known as Snell's law of refraction.
(b) The path of rays is as shown in figure.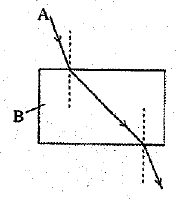
|
80 videos|569 docs|80 tests
|





















