Class 10 Science: CBSE Sample Question Paper (2019-20) - 9 | Science Class 10 PDF Download
Class-X
Science-086
SAMPLE QUESTION PAPER 2019-20
TIME: 3 Hrs.
M.M: 80
General Instructions:
1. The question paper comprises three sections - A, B and C. Attempt all the sections.
2. All questions are compulsory.
3. Internal choice is given in each section.
4. All questions in Section A are one - mark questions comprising MCQ, VSA type and
assertion-reason type questions. They are to be answered in one word or in one sentence.
5. All questions in Section B are three - mark, short - answer type questions. These are to be answered in about 50 - 60 words each.
6. All questions in Section C are five - mark, long - answer type questions. These are to be answered in about 80 - 90 words each.
7. This question paper consists of a total of 30 questions.
Section A
Q.1. Should the resistance of a voltmeter be low or high ? Give reason. (1 Mark)
Ans: High
In parallel connection, less current passes through high resistance.
Q.2. Why should curd and sour substances not be kept in brass and copper vessels ? (1 Mark)
Ans: Curd and sour substances are acidic in nature. The acid present in curd and sour substances react with metal surface of brass and copper vessels to produce toxic compounds which are not fit for human consumption. In short, we can say that, 'it causes toxicity of food'.
Q.3. What is the resistor value in the given circuit ? (1 Mark)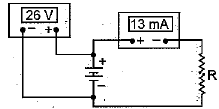
(a) 200 Ω
(b) 1 kΩ
(c) 2 kΩ
(d) 4 kΩ
Ans: (c) Value of resistor 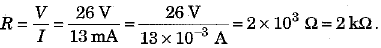
Q.4. For Q. Nos. (i)-(iv) are based on the information given below. Study these information related to answer the questions that follow :
Adrenaline hormone is released in the body during stress or emergency situations. It generates several responses which together enable the body to deal with a situation. Given below is a table related to the possible effects of adrenaline hormone on liver glycogen and blood as a result of increased adrenaline secretion glucose.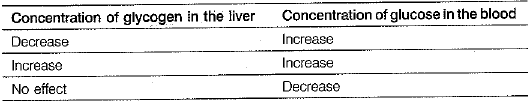
(i) Identify the gland from which the adrenaline hormone is secreted. (1 Mark)
(ii) Which one of the following is an affect of adrenaline hormone in an individual ? (1 Mark)
(a) Increased rate of breathing
(b) Increased muscle strength
(c) Decreased metabolism of fats (d) Both (a) and (b)
(iii) Adrenal gland is which type of gland? (1 Mark)
(a) Endocrine
(b) Exocrine
(c) Paracrine
(d) None of these
(iv) Which of the following options given in table above correctly despicts the effect of adrenaline on blood glucose? (1 Mark)
Ans: (i) Adrenal gland.
(ii) (d) Adrenaline secretion increases the rate of heart beats and muscle strength.
(iii) (a) Adrenal gland secretes hormone adrenaline directly into the blood from where it is carried to different body parts. Hence, it is an endocrine gland.
(iv) Adrenaline increases the blood glucose level. This is achieved by increasing the rate of conversion of glycogen to glucose in liver and muscles.
Q.5. Which of the following gives the correct increasing order of the atomic radii of O,F and N? (1 Mark)
(a) O,F,N
(b) N,F,O
(c) O,N,F
(d) F,O,N
Ans: (d) In the Modern Periodic Table, the atomic radii decrease with increasing the atomic number from left to right. The atomic number of F, O, and N are 9, 8, and 7, respectively so atomic radius will decrease from N to F.
Q.6. An electric bulb is rated 220 V and 100 W.
When it is operated on 110 V, the power consumed will be : (1 Mark)
(a) 100 W
(b) 75 W
(c) 50 W
(d) 25 W
Ans: (d) 25 W
Q.7. Aqua regia that is capable of dissolving Ag and Au is a mixture of HCl and HNO3 respectively in the ratio of (1 Mark)
(a) 3 : 1
(b) 1 : 3
(c) 2 : 1
(d) 1 : 2
Ans: (a) 3 : 1
Q.8. Splitting of white light into seven colours on passing through a glass prism is called (1 Mark)
(a) scattering
(b) reflection
(c) dispersion
(d) refraction
Ans: (c) The phenomenon of splitting of white light into its constituent colours after passing through a prism is called dispersion.
Q.9. The danger signals installed at the top of tall buildings are red in colour. These can be easily seen from a distance because among all other colours, the red light: (1 Mark)
(a) is scattered the most by smoke or fog
(b) is scattered the least by smoke or fog
(c) is absorbed the most by smoke or fog
(d) moves fastest in air
Ans: (C) The optical device is a concave mirror. It is because when light rays from a distant object (here it is Sun) fall on this mirror, its image is formed at the focus of the mirror on the same side as the object. Thus, in the given case, distance between the mirror and the screen is the focal length of the concave mirror. Hence, the focal length of the given device (concave mirror) is 24 cm.
Q.10. A Mendelian experiment consisted of breeding tall pea plants bearing violet flowers with short pea plants bearing white flowers. The progeny all bore violet flowers, but almost half of them were short. This suggests that the genetic make-up of the tall parent can be depicted as : (1 Mark)
(a) TTWW
(b) TTww
(c) TtWW
(d) TtWw.
Ans: (c) TtWW.
Q.11. 1 kw h = __________J (1 Mark)
Ans: 3.6 x 106
Q.12. Which one of the following components help in the recycling of materials in an ecosystem? (1 Mark)
(a) autotrophs
(b) saprotrophs
(c) omnivores
(d) carnivores
Or
Food cans are coated with tin and not with zinc beacuse
(a) zinc is costlier than tin
(b) zinc has a higher melting point than tin
(c) zinc is more reactive than tin
(d) zinc is less reactive than tin
Ans: (b) Saprotrophs (or decomposers) breakdown the complex organic compounds in dead matter into simpler forms and help in recycling of materials in an ecosystem.
Or
Food cans are not coated with zinc because it being more reactive than tin, can react with organic acids present in the food.
Q.13. For question numbers 13 and 14, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (i), (ii), (iii) and (iv) as given below:
(i) Both A and R are true and R is correct explanation of the assertion.
(ii) Both A and R are true but R is not the correct explanation of the assertion.
(iii) A is true but R is false.
(iv) A is false but R is true.
Assertion (A): Unisexual flowers have separate male and female flowers.
Reason (R): Cucumber, pumpkin and watermelon are example of unisexual flowers.
OR
Assertion (A): Units which make up the nervous system are called neurons.
Reason (R): Nerve impulses are carried by dendrites towards the cell body. (1 Mark)
Ans: (b) Unisexual flowers have separate male and female flowers. The example includes cucumber, pumpkin and watermelon.
OR
(b) Both the statements are true. Nervous system is the system of conducting tissues that receives the stimulus and transmits it to other parts of the body forming a network of nerves. It is involved in receiving information (sensation) and generating responses to that information (motor response). The units which make up the nervous system are called nerve cells or neurons. Nerve impulses are always transmitted across a synapse from the axon terminals of one neuron to the dendrite /cell body of the next neuron.
Q.14. For question numbers 13 and 14, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (i), (ii), (iii) and (iv) as given below (1 Mark)
(i) Both A and R are true and R is correct explanation of the assertion.
(ii) Both A and R are true but R is not the correct explanation of the assertion.
(iii) A is true but R is false.
(iv). A is false but R is' true.
Assertion : Laws of refraction are valid for all angles on incidence.
Reason: When a.ray of light falls perpendicular on the interface separating two transparent surfaces, it does not undergo refraction.
(i) A
(ii) B
(iii) C
(iv) D
Ans:(iv)
Section B
Q.15. Read the Assertion and Reason carefully and then mark the correct option out of the options given below : (3 Mark)
(a) If both assertion and reason are true and the reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but reason is false.
(d) If the assertion and reason both are false.
Assertion : All the organic compounds that are obtained from plant and animal sources are being synthesised these days.
Reason : In saturated hydrocarbons, four valencies of carbon are satisfied with the help of single bonds.
Ans: (b)
Q.16. Identify the acid and base which form sodium hydrogen carbonate. Write chemical equation in support of your answer. State whether this compound is acidic, basic or neutral. Also, write its pH value.
OR
What are amphoteric oxides ? Give an example.
Write balanced chemical equations to justify your answer. (3 Mark)
Ans:
Acid - H2O3
Base - NaOH
NaOH + H2CO3 → NaHCO3 + H2O
Compound is basic in nature.
pH value - ranges between 7 to 10.
OR
Metal oxides showing both acidic and basic nature.
Example: Al2O3/ZnO (or any other)
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Q.17. Three elements 'X', 'Y' and ‘Z’ having atomic numbers 11, 7 and 6 respectively react with oxygen to form their oxides.
(a) Arrange these oxides in increasing order of their basic nature.
(b) Give reason for your answer. (3 Mark)
Ans: 11X = 2,8,1
7Y = 2,5
6Z = 2, 5
(a) Y< Z< X
(b) X is metallic in nature hence, its oxide is basic in nature. While Y and Z are non-metals and their oxides are acidic in nature.
(In a period, acidic character and basic character decreases.)
Q.18. List four functions of the human heart. Why is double circulation necessary in the human body ? (3 Mark)
Ans: Four functions of human heart are :
(i) It receives deoxygenated blood from the body for oxygenation.
(ii) Sends the blood to the lungs for oxygenation.
(iii) Receives oxygenated blood from the lungs to be supplied to body.
(iv) Sends oxygenated blood to various body parts, so that each cell gets energy.
Double circulation is necessary for human body. It ensures quick and efficient supply of oxygenated blood to all body parts for meeting higher energy needs and for thermoregulation of body in mammals and birds.
Q.19. 'Reproduction is not necessary to maintain the life of an individual, unlike other life processes such as nutrition, respiration or excretion. Still, an individual wastes energy for reproduction. Derive your own conclusion from the above given statement and describe it accordingly. (3 Mark)
Ans: Reproduction is the ability of a living organism to produce new individuals similar to them. Like other essential life processes (nutrition, respiration, growth and excretion), reproduction is not essential to maintain the life of an individual. However, it is essential for the purpose of
• continuation of life on the Earth.
• addition of new species.
• replacement of dead organisms.
• transfer of variations from one generation to another.
Q.20. What happens to a beam of white light when it gets refracted through a glass prism ? Which colour deviates the most and the least after refraction through a prism ? What is likely to happen if a second identical prism is placed in an inverted position with respect to the first prism ? Justify your answer.
OR
A student needs spectacles of power - 0.5 D for the correction of his vision.
(i) Name the defect in vision the student is suffering from.
(ii) Find the nature and focal length of the corrective lens.
(iii) List two causes of this defect. (3 Mark)
Ans: The white light splits into seven colours when it gets refracted through the glass prism (VIBGYOR).
The colour deviates most - Violet
The colour deviates least - Red
Colours disappear and again white light is obtained.
Q.21. Name the hormone synthesised at the shoot tips. How does it help the plant to respond to light ? (3 Mark)
Ans: Auxin is synthesised at the shoot tips when growing plant detects light and helps the cells to grow longer.
When light is coming from one side of the plant, auxin diffuses towards the shady side of the shoot. This concentration of auxin stimulates the cells to grow longer on the side of the shoot which is away from light. Thus, the plant appears to bend towards light.
Q.22. List in tabular form three distinguishing features between cerebrum and cerebellum. (3 Mark)
Ans: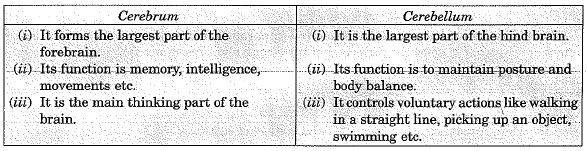
Q.23. A household uses the following electric appliances.
(i) A heater of rating 1000 W for 5 hours in each day.
(ii) 5 electric bulbs of rating 100 W each for 5 hours in each day (given, cost of electrical energy Rs. 2.5 per unit).
Find the electricity bill for the household for the month of April. (3 Mark)
Ans: Total number of units consumed by heater for 5 hours each day in one month,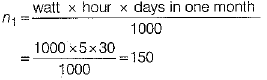
Cost of electrical energy consumed by heater in month of April = 150 x 2.5 = Rs. 375
Total number of units consumed by 5 electric bulbs in one month,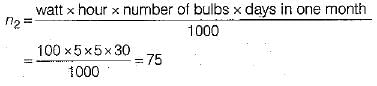
Cost of electrical energy consumed by 5 bulbs in one month = 75 x 2.5 = Rs. 187.5
Electricity bill for the month of April = Rs. 375 +Rs. 187.5 = Rs. 562.5
Alternate solution
Total power consumed by electric appliances (heater and 5 bulbs) per day,
P = 1000 + 100 x 5
= 1500 watt
Total number of hours of operation of electric appliances in a month of April,
= 5 x 30 = 150 hours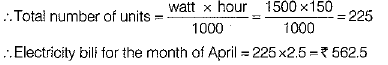
Q.24. What are solar cells ? Explain the structure of solar panel. List two principal advantages associated with solar cells. (3 Mark)
Ans: Solar cells are the devices that convert solar energy of Sun into electricity.
Solar panel consists of a large number of solar cells connected by silver wires. They are made up of special grade silicon. These are mounted on specially designed inclined roof tops so that more solar energy is incident over it.
Advantages of solar cells :
(a) They have no moving parts, require little maintenance and work quite satisfactorily without the use of any focussing device.
(b) They can be set up in remote and inaccessible hamlets or very sparsely inhabited areas.
Section C
Q. 25. Define a chemical reaction. State four observations which help us to determine that a chemical reaction has taken place. Write one example of each observation with a balanced chemical equation. (5 Mark)
Ans: Process in which new substances with new properties are formed by the rearrangement of atoms.
(i) Evolution of gas: The chemical reaction between zinc and dilute H2SO4.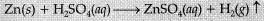
(ii) Change in colour: The chemical reaction between potassium iodide and lead nitrate.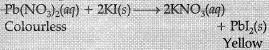
(iii) Formation of precipitate: The chemical reaction between sulphuric acid and barium chloride.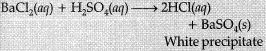
(iv) Change in temperature: The chemical reaction between quick lime and water.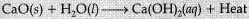
Q. 26. A compound C (molecular formula, C2H4O2) reacts with Na- metal to form a compound R and evolves a gas which bums with a pop sound. Compound C on treatment with an alcohol A in presence of an acid forms a sweet smelling compound S (molecular formula, C3H6O2). On addition of NaOH to C, it also gives R and water. S on treatment with NaOH solution gives back R and A.
Identify C, R, A, S and write down the reactions involved. (5 Mark)
Ans: C — Ethanoic acid.
R — Sodium salt of ethanoic acid (sodium acetate) and gas evolved is hydrogen.
A — Methanol.
S — Ester (Methyl acetete).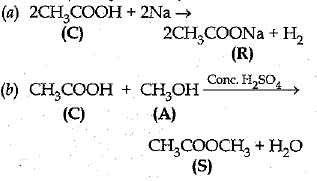
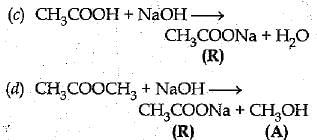
Q. 27. (a) Name and state the rule to determine the direction of force experienced by a current carrying straight conductor placed in a uniform magnetic field which is perpendicular to it.
(b) Draw a labelled diagram of an electric motor. (5 Mark)
Ans: (a) Direction of force acting on a current- carrying straight conductor due to a uniform perpendicular/magnetic field can be easily found by applying Fleming’s left-hand rule.
Fleming’s left-hand rule states that stretch the forefinger, the central finger and the thumb of your left-hand in mutually perpendicular directions. If the forefinger shows the direction of the magnetic field and the central finger that of the current, then the thumb wall point in the direction of force F.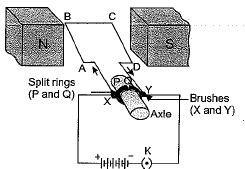
(b) A labelled diagram of an electric motor is drawn here.
Q. 28. (i) Bacteria have a simpler body plan as compared to human beings who have a complex body organisation. Does it mean that human beings are more evolved than bacteria?
(ii) How can you conclude that birds are closely related to reptiles?
Or
(i) Study the following cross showing self-pollination in pea plant.
Fill in the blanks and answer the questions that follows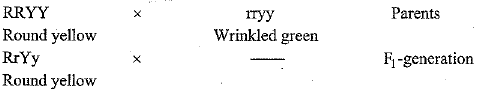
(ii) Derive the combinations of characters expected in the F2 progeny? Also write the ratios,
(iii) According to you, what is responsible for the inheritance of traits? (5 Mark)
Ans: (i) The terms simple and complex are used to classify organisms according to their evolutionary history. Simple organisms refer to those organisms that have simple structural and functional organisation and are considered primitive, whereas complex organisms refer to those organisms that have higher and complex levels of structural and functional organisation. These are more advanced and said to have arisen from simple organisms. Both bacteria and human beings perform all the activities of life in order to persist in their respective environment. Human beings have a more complex organisation and . differentiation. These features are absent in bacteria. Since, complexity and differentiation develop only through evolution, humans are said to be more evolved than bacteria.
(ii) Feathers provided insulation to dinosaurs in cold weather. Later in the evolutionary process, feathers were used for flight in birds. As dinosaurs were reptiles, it means that birds are closely related to reptiles.
Or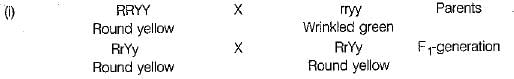
(ii)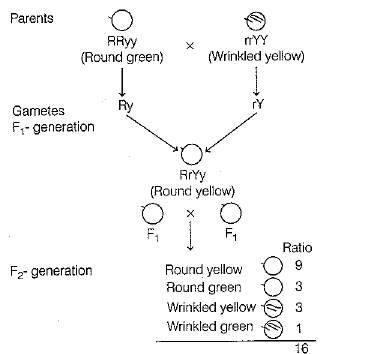
Thus, the combination of characters obtained in the F2-generation and their respective ratios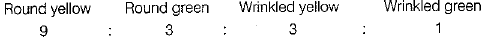
(iii) Gene is the carrier that leads to the inheritance of traits. It is the part of a chromosome that controls the appearance of a set of hereditary characteristics.
Q.29. What is a solenoid? Draw the pattern of magnetic field lines of (i) a current carrying solenoid and (ii) a bar magnet. List two distinguishing features between the two fields. (5 Mark)
Ans: A solenoid is a coil of insulated copper wire consisting of a large number of turns closely wrapped together very tightly into a shape of a cylinder.
(i) Pattern of magnetic field lines of a current carrying solenoid: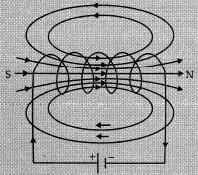
(ii) Pattern of magnetic field lines around a bar magnet: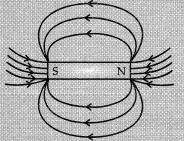
Two distinguishing features between the tow fields:
(i) Magnetic field of solenoid can be changed according to our requirements by just changing current or core of solenoid while the magnetic field of bar magnet is fixed.
(ii) Magnetic field outside the solenoid is negligible in comparison to the bar magnet.
Q. 30. An object is placed at the following distances from a concave mirror of focal length 10 cm:
(a) 8 cm
(b) 15 cm
(c) 20 cm
(d) 25 cm.
Which position of the object will produce ?
(i) a diminished real image?
(ii) a magnified real image?
(iii) a magnified virtual image.
(iv) an image of the same size as the object?
Or
(a) If a person wears lens of power - 6 D for distant vision and for correcting his near vision he needs a lens of +2 D. Determine the focal length of the lenses in both the case.
(b) Give reason for the following natural phenomenon:
(i) Stars twinkle
(ii) Planets do not twinkle
(iii) Stars appear raised in the sky (5 Mark)
Ans: Given focal length of mirror = 10 cm Therefore, distance of centre of curvature - 20 cm
(i) A diminished real image is formed by a concave mirror, when the object lies beyond C. Here C is at 20 cm. Therefore, the diminished real image will be formed when the object is at a distance greater than 20 cm, which in this problem is 25 cm. Hence, the position of the object for a diminished real image is 25 cm.
(ii) A magnified real image is formed by a concave mirror when the object is between F and C. Here F is at 10 cm and C is at 20 cm. Hence, the magnified real image will be formed when the object lies between 10 cm and 20 cm which in this problem is 15 cm. Therefore, the position of object for a magnified real image is 15 cm.
(iii) A concave mirror forms a magnified virtual image, when the object is within focus (F) at a distance less than focal length or less than 10 cm, which in this problem is 8 cm. Therefore, the position of object for a magnified virtual image is 8 cm
(iv) An image of the same size as object is formed by a concave mirror when the object lies at the centre of curvature (c). Here C is at 20 cm, therefore, the image of the same size as the object will be formed when the object is at 20 cm from the concave mirror. Thus, the position of the object for an image of same size as the-object is 20 cm.
Or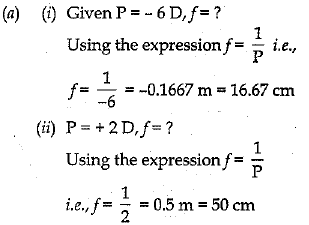
(b) (i) The layers of the earth's atmosphere have different densities and hence different refractive index. This refractive index keeps on changing due to conventional currents in the atmosphere. When light from the star which can be approximated as a point source of light, enters such an atmosphere it seems to change its' position. The light coming from the stars, therefore, presents a quivering (shaking) appearance, thus, giving the impression as if the stars are twinkling.
(ii) The stars, although very large as compared to the planets, appear as tiny specks in the sky due to their immense distance from the earth as compared to the huge size of the planets. Thus, the stars appear as point sources of light and the planets appear as a large combination of such sources. Due to its seemingly larger size, the change in the direction of light coming from the planets is negligible as compared to that coming from the stars. Therefore, the planets do not seem to twinkle like stars.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper (2019-20) - 9 - Science Class 10
| 1. What is the importance of solving CBSE sample question papers for Class 10 Science? |  |
| 2. How can CBSE sample question papers for Class 10 Science be used effectively for exam preparation? |  |
| 3. Are the questions in CBSE sample question papers for Class 10 Science similar to the actual exam? |  |
| 4. How can I score well in the Class 10 Science exam using CBSE sample question papers? |  |
| 5. Can CBSE sample question papers for Class 10 Science be used for self-assessment? |  |
















